Disclaimer on Environmental Justice
As a result of E.O. 14148, E.O. 14154, E.O. 14173, and the removal of the Council on Environmental Quality's regulations, all federal environmental justice requirements are revoked and no longer applicable to the federal environmental review process. Any purported environmental justice impacts will not be considered in the federal decision. Social, economic, and community impacts will continue to be disclosed where applicable in accordance with 23 CFR 771.
Literature Review: Pollinator Habitat Enhancement and Best Management Practices in Highway Rights-of-Way
Download the PDF
Prepared for:
The Federal Highway Administration
1200 New Jersey Avenue, SE
Washington, D.C. 20590
Prepared by:
The Xerces Society for Invertebrate Conservation
in collaboration with
ICF International
Authors: Jennifer Hopwood, Scott Hoffman Black, Eric Lee-Mader, Alexandra Charlap, Robert Preston, Kailash Mozumder, Scott Fleury
May 2015
ICF International and the Xerces Society for Invertebrate Conservation prepared this literature review as part of a contract to U.S. Department of Transportation,
Federal Highway Administration. This literature review is for general information only and the statements, findings, conclusions and recommendations are
those of the author(s) and do not represent the policies or positions of FHWA or the U.S. Department of Transportation.
Contents
List of Tables and Figures
List of Acronyms and Abbreviations
Executive Summary
Chapter 1 Introduction and Purpose
1.1 Background
1.2 Research and Report Development Process
1.3 Identifying and Reviewing Documents
1.4 Benefits of Roadsides to Pollinators
Chapter 2 Pollinator Science and Conservation Issues
2.1 Important Pollinator Groups
2.2 Pollinator Decline
2.3 Benefits of Roadsides to Pollinators
2.3.1 Ecological Functions Provided by Roadsides
Chapter 3 Threats to Pollinators Associated with Roads and Roadsides
3.1 Roadside Vegetation Management
3.1.1 Mowing
3.1.2 Herbicides
3.1.3 Grazing
3.1.4 Fire
3.1.5 Biological Control
3.2 Mortality Due to Traffic
3.3 Roads and Habitat Modification
3.3.1 Roads and Habitat Fragmentation
3.3.2 Roads as Potential Barriers to Pollinator Movement
3.3.3 Introduced Plant Species.
3.3.4 Roadside Contamination.
3.3.5 Pesticide Drift from Adjacent Land
Chapter 4 Restoring Habitat for Pollinators
4.1 Considerations for Pollinator Habitat Restoration
4.2 Pollinator Habitat Restoration Planning and Design
4.2.1 Site Inventory
4.2.2 Restoration Design
Chapter 5 Vegetation Management for Pollinators
5.1 Managing Roadside Vegetation with Mowing
5.2 Managing Roadside Vegetation with Herbicides
5.3 Managing Roadsides by Establishing Native Vegetation
5.4 Use of Fire on Roadsides
5.5 Use of Grazing on Roadsides
5.6 Use of Biological Control on Roadside Weeds
5.7 Incorporating All the Tools: Integrated Roadside Vegetation Management
Chapter 6 Case Studies
6.1 Bringing prairie back to Iowa: Iowa’s Integrated Roadside Vegetation Management Program and Living Roadway Trust Fund
6.2 Monarch-Friendly Roadside Management: Roadsides for Wildlife Program, Minnesota
Chapter 7 Literature Cited
7.1 Printed References
7.2 Personal Communications
Back to top
Tables and Figures
Table 1 - General Restoration Goals to Meet Pollinator Habitat Requirements
Figure 1 - Area of Forest Occupied by Colonies of Hibernating Monarchs in Mexico
Figure 2 - Monarch Estimates at California Overwintering Sites from 1997 to 2013
Acronyms and Abbreviations
|
BMPs
|
best management practices
|
|
DNR
|
Department of Natural Resources
|
|
DOT
|
Department of Transportation
|
|
FHWA
|
Federal Highway Administration
|
|
ICF
|
ICF International
|
|
IPM
|
Integrated Pest Management
|
|
IRVM
|
Integrated Roadside Vegetation Management
|
|
km
|
kilometers
|
|
m
|
meters
|
|
NRCS
|
National Resources Conservation Service
|
|
ROW
|
right-of-way
|
Back to top
Executive Summary
Pollinator services are “central to all human beings, livestock, and wildlife” (Kevan 1999). Plant pollination by insects is one of the most
well-known and important ecosystem services and is essential in both natural and agricultural landscapes. An estimated 85 percent of the world’s
flowering plants depend on animals—mostly insects—for pollination (Ollerton et al. 2011). Eighty-seven of the world’s 124 most commonly
cultivated crops (70 percent) are reliant on animal pollinators, and insect-pollinated forage plants such as alfalfa and clover provide feed for livestock
(Klein et al. 2006). Roughly 35 percent of global crop production is dependent on pollination by animals (Klein et al. 2006). Pollinators also sustain
wildland plant communities that provide food and shelter for myriad other wildlife and contribute to overall ecosystem health.
The great majority of pollinators are insects, including bees, wasps, flies, beetles, butterflies, and moths (Speight 1978; Allen-Wardell et al. 1998;
Jennersten 1988; Frankie et al. 1990; Irvine and Armstrong 1990; Kevan 1999; Westerkamp and Gottsberger 2000; Kearns 2001; Larson et al. 2001), but many
bird and bat species pollinate as well (Grant 1994; Valiente-Banuet et al. 2004). Bees are considered the most important group of pollinators for agricultural
crops as well as for wild plants in temperate climates (Morse and Calderone 2000; Garibaldi et al. 2013).
The domesticated European honey bee (Apis mellifera L.) is the most widely managed crop pollinator in the United States. Studies indicate that
honey bees are important for more than $15 billion in crop production annually (Morse and Calderone 2000; Calderone 2012).
There are also approximately 4,000 species of native bees in North America (Michener 2007), and they are also important crop pollinators (e.g., Tepedino
1981; Bosch and Kemp 2001; Javorek et al. 2002; Winfree et al. 2008; Garibaldi et al. 2013). Native bees are important in the production of an estimated
$3 billion worth of crops annually to the United States economy (Losey and Vaughan 2006; Calderone 2012), and emerging research shows that this is likely
an underestimate of their total value (Garibaldi et al. 2013).
There is evidence of declines in both domesticated and wild pollinators. The number of honey bee colonies has been in decline over the past half-century
because of disease, parasites, lack of floral resources, insecticides, and other factors (National Research Council 2007). Since 2006, beekeepers have
experienced record high annual hive losses of 29 percent or more (Bee Informed Partnership 2014), and several species of once-common bumble bees have
become rare (Cameron et al. 2011).
Other pollinators are also in decline. Monarch butterfly populations have dropped by 90 percent east of the rocky mountains (Rendon-Salinas and Tavera-Alonso
2014 ) and by 50 percent west of the Rockies (Monroe et al. 2014 ), and other butterfly species have also seen significant declines. NatureServe (a primary
source for species conservation data, status, and trends in the United States) has assessed all 800 butterfly species in the United States and has found
that 141 (17 percent) are at risk of extinction (NatureServe 2014 ). Twenty-six species of butterflies are listed as threatened or endangered under the
Federal Endangered Species Act (U.S. Fish and Wildlife Service 2014 ).
Factors leading to decline include habitat loss, pesticide use, diseases, parasites, and the spread of invasive species. Threats to pollinator communities
affect not only pollinators themselves but also natural ecosystems and agricultural productivity.
Threats to pollinators specifically associated with roads include mortality due to vehicle collisions, habitat fragmentation, barriers to movement, effects
of roadside vegetation management, exposure to invasive plants, drift from adjacent land, and pollution from vehicles. Despite these threats, roadsides
are a conservation opportunity to increase pollinator habitat.
Roadsides can provide habitat for a diverse community of pollinators. Roadside habitat can include forage for food and breeding or nesting opportunities.
Roadsides extend across a variety of landscapes and can aid dispersal of pollinators by linking fragmented habitats. By acting as refugia for pollinators
in otherwise inhospitable landscapes, roadside habitat can contribute to the maintenance of healthy ecosystems and provision of ecological services such
as crop pollination services.
Back to top
Chapter 1: Introduction and Purpose
The objective of this literature review is to establish a foundation for the development of best management practices (BMPs) for pollinator habitat protection
and enhancement in highway rights-of-way (ROWs) that will be described in two subsequent reports: (1) a high-level technical report for Federal Highway
Administration (FHWA) and States’ Departments of Transportation (DOT) program and policy staff; and (2) a detailed BMP guidance document for field
applications by State DOT field staff and contractors. This document represents a first step in an effort to provide practicable BMPs that FHWA can offer
transportation agencies to develop pollinator programs in their jurisdictions and enhance these programs where they already exist. This document does
not include the FHWA BMPS, which are still under development.
Background
In July 2014, FHWA awarded ICF International (ICF) a contract to develop pollinator BMPs for promoting pollinator habitat protection and enhancement in
highway ROW. ICF has partnered with the Xerces Society to assist in the preparation of these BMPs. On September 17, 2014, a work plan was approved by
FHWA. Task 2 of this contract and work plan calls for the development of a thorough literature review of this topic. FHWA has accepted this literature
review as satisfying the requirements of Task 2, and approved ICF and Xerces ongoing work towards the development of BMPs to benefit pollinators.
Research and Report Development Process
Xerces Society and ICF staff performed a thorough literature review of peer-reviewed and technical material on the topic of vegetation management to enhance
pollinator habitat. We researched and summarized the literature within the context of the broader literature on pollinator decline, the causes, and mitigation
of stressors. The review includes a comprehensive treatment of habitat restoration and management for pollinators including bees (both native bees and
the domesticated European honey bee), butterflies, and vertebrate pollinators such as hummingbirds and nectar-feeding bats. We focus special attention
on key declining pollinators such as agriculturally important bumble bees and the iconic monarch butterfly, because roadsides can be of special importance
to their conservation. We specifically look at how this body of information can be applied in highway ROWs while considering feasibility relative to existing
practices, guidelines, and budgetary limits.
Identifying and Reviewing Documents
We reviewed existing Xerces Society library documents related to pollinators, restoration, and management, supplemented by academic library research of
search engines to locate articles using keyword searches. We also contacted our colleagues at Federal and State resource agencies, including State DOTs,
to request specific information in the unpublished grey literature (reports on pollinator and vegetation management of roadsides, farmland, and natural
landscapes).
In reviewing these materials, we identified other appropriate information resources and queried colleagues to make sure information in publications that
are in preparation or in press were also included. Information from all of the above sources was reviewed and summarized, and citations were compiled.
Benefits of Roadsides to Pollinators
Roadsides cover more than 10 million acres of land in the United States (Forman et al. 2003), stretching across agricultural and urban landscapes. Though
roadsides are not a substitute for wildlands, they can be valuable habitat for wildlife, acting as linear refuges and connecting remnant habitat patches.
In highly modified landscapes, roadsides may be the only seminatural habitat remaining, and with 4 acres of open space lost to development every minute
(U.S. Department of Agriculture, Forest Service 2006), roadsides provide an opportunity to contribute to efforts to conserve and manage pollinators and
their habitats.
Research has shown that the maintenance of native wildflowers on roadsides is beneficial to pollinators. In Kansas, Hopwood (2008) found bees to be twice
as abundant on roadsides with native plants compared with those dominated by nonnative grass and flowers. Similarly, roadsides with native plants were
found to support about 35 percent more bee species. Butterflies also benefit from the presence of native plants on roadsides, as shown by many North American
and European studies (Ries et al. 2001).
Mowing, herbicide use, grazing, and other roadside management strategies can be done in a way to promote pollinators and their habitat (e.g. Noordijk
et al. 2009). Research shows that the restoration and management of roadsides to benefit a broad suite of pollinators can also be compatible with current
Integrated Roadside Vegetation Management (IRVM) practices (e.g. Ries et al. 2001). By encouraging plant diversity and targeting mowing and herbicide
use, IRVM improves the quality of the roadside habitat. IRVM practices that limit disturbance but maintain plant diversity such as spot mowing to reduce
weed seed production, limited grazing, occasional prescribed fire, and biological control likely also benefit pollinators. Plant species selection designed
for IRVM plantings can fulfill functional roles valuable to roadside vegetation management while also supporting pollinators (e.g. Brandt et al. 2011).
The following sections summarize the literature regarding key issues of pollinator conservation and management on roadsides, including importance to agriculture
and ecosystem function, decline in important pollinator groups, factors leading to decline, restoration and management strategies for pollinators, and
compatibility with restoration and current road management.
Back to top
Chapter 2: Pollinator Science and Conservation Issues
2.1 Important Pollinator Groups
Animal pollinators in North America include bees, butterflies, moths, wasps, flies, beetles, ants, bats, and hummingbirds. Insects make up the vast majority
of pollinator species, and bees are the most important pollinators in temperate North America (Morse and Calderone 2000; Garibaldi et al. 2013).
The nonnative honey bee (Apis mellifera) is the most widely recognized and managed crop pollinator in the United States. Studies indicate that
honey bee pollination accounts for more than $15 billion in crop production annually (Morse and Calderone 2000; Calderone 2012).
There are approximately 4,000 species of native bees in North America (Michener 2007), many of which are also important crop pollinators. Native bees
are important in the production of an estimated $3 billion worth of crops annually to the United States economy (Losey and Vaughan 2006; Calderone 2012).
A recent survey found that native bees universally increased fruit set in 41 crop systems worldwide, independent of honey bee presence (Garibaldi et al.
2013). In the same study, honey bees only increased fruit set in 14 percent of the same 41 systems while, overall, native bees enhanced fruit set by twice
as much as an equivalent increase in honey bee visitation. Native bees provide free pollination services and are often more efficient than honey bees
on an individual bee basis at pollinating particular crops, such as squash, berries, and tree fruits (e.g., Tepedino 1981; Bosch and Kemp 2001; Javorek
et al. 2002; Garibaldi et al. 2013).
Bumble bees have many qualities that contribute to their suitability as agricultural pollinators. They are able to fly in cooler temperatures and lower
light levels than many other bees, which extends their work day and improves the pollination of crops during inclement weather (Corbet et al. 1993). They
also possess the ability to “buzz pollinate,” in which a bee grabs the pollen producing structure of the flower in her jaws and vibrates her
wing musculature. This activity causes the flower to vibrate, which in turn dislodges pollen that would have otherwise remained trapped in the flower’s
anthers (Buchmann 1983). Some plants, including blueberries, cranberries, tomatoes, and peppers, are specially adapted to benefit from buzz pollination,
which few bees other than bumble bees can provide. Bumble bees have been shown to be an excellent pollinator of cranberry (Cane and Schiffauer 2003) and
other important food crops such as plum and apple (Medler and Carney 1963; Mitchell 1962), alfalfa (Holm 1966), and onion for seed production (Caron et
al. 1975).
In addition to commercially important crops, bumble bees also play a vital role as generalist pollinators of native flowering plants, helping to maintain
plant communities that support the pollinator fauna. An examination of the theoretical effect of removing specialist and generalist pollinators on the
extinction of plant species concluded that the loss of generalist pollinators, especially bumble bees, leads to the greatest number of plant extinctions
(Memmott et al. 2004). In Britain and the Netherlands, where multiple pollinators have declined, there is evidence of a parallel decline in the abundance
of insect-pollinated plants (Biesmeijer et al. 2006).
Of the other orders of pollinating insects, flies (Diptera) also provide substantial pollination services (Speight 1978; Kearns 2001; Larson
et al. 2001), especially in alpine areas and tundra. Other insects such as beetles (Coleoptera) and wasps (Hymenoptera) provide pollination
services, though to a lesser extent (e.g., Frankie et al. 1990; Irvine and Armstrong 1990; Kevan 1999). Most butterfly and moth species (Lepidoptera)
visit flowers for nectar, although their contribution to pollination services is unknown (Jennersten 1988; Frankie et al. 1990; Allen-Wardell et al. 1998;
Westerkamp and Gottsberger 2000). Many butterfly species take long flights between flowers and may carry pollen for a long time; therefore, in this respect,
they may be effective as dispersers of pollen, but there are limited studies on this topic. Some flowering plants are specially adapted for butterfly
pollination. The vibrant firecracker plant (Russelia spp.) is pollinated by the orange barred sulfur butterfly (Phoebis philea);
its weeping branches cause the flowers to hang in such a way that makes it difficult for other insects to pollinate. Flowers of many species of Phlox,
Lantana, and Zinnia are also pollinated primarily by butterflies. The Apollo Parnassian (Parnassius apollo) pollinates Senecio
and other composites, its hairy body easily picking up and distributing pollen. Monarch butterflies (Danaus plexippus) likely pollinate some
milkweeds and have been found with milkweed pollen bundles, called pollinia, hanging from their legs (personal communication, Dr. Karen Oberhauser, University
of Minnesota ).
In addition to insect pollinators, there are two groups of nectar-feeding (nectarivorous) vertebrates that play an important role in pollination: hummingbirds
and bats. Both of these groups fall under subfamilies that have developed specialized morphological, physiological, and behavioral traits that allow them
to feed primarily on the nectar of plants (Gonzalez-Terrazas et al. 2012). These traits include characteristics like smaller body size and wing morphology
that allow for extended periods of hovering, elongated tongues, and specialized mouth/bill shapes for efficient nectar consumption. Similarly, many of
the plants that these vertebrate groups feed on have unique flower characteristics and pollination strategies that have co-evolved with these vertebrate
pollinators (Arita and Wilson 1987).
The North American nectar-feeding bats are members of the leaf nosed family Phyllostomidae, subfamily Glossophaginae (Wilson and Reeder 2005), including
12 species that are known pollinators in North America and Mexico (National Research Council 2007). These bats make up a small but important group of
the 45 total species of bats that occur in the United States. The largest concentrations of nectar-feeding bats occur in the deserts of Arizona, California,
Nevada, New Mexico, and Texas. The majority of the information known about these nectar-feeding bats is derived from studies of the lesser long-nosed
bat (Leptonycteris curasoae), the Mexican long-nosed bat (Leptonycteris nivalis), and the hog-nosed bat (Choeronycteris mexicana).
The known range for these bat species corresponds closely with the distribution of columnar cacti (e.g., saguaro [Carnegiea gigantea], Pachycereus
spp., Stenocereus spp., Lophocereus spp.) and agaves (Agave spp.), the primary species they are known to pollinate (Valiente-Banuet
et al. 2004). Columnar cacti and agaves are long-lived plants that are often the dominant structures in their arid ecosystems, where they provide food
and shelter for a range of species including birds, bats, mammals, and insects. This ecologically important role is heavily reliant on the pollination
and seed dispersal that is provided by nectar-feeding bats. Many of the plant species that rely on pollinating bats have developed specific floral characteristics
to facilitate bat pollination. The two primary characteristics shared across plant species pollinated by bats are large flower and/or inflorescence that
extend away from the plant’s foliage and nocturnal blooming often associated with a strong musty odor and increased nectar production (Fleming et
al. 2009).
Pollination by bird species is also important and often exemplified by the numerous species of hummingbirds that occur in North America. These hummingbird
species make long migratory journeys (some for thousands of miles) and depend on nectar corridors to meet the energy demands they undergo to sustain their
long-distance movements (Nabhan et al. 2004). These nectar corridors have become established over long periods of time, as evidenced by the mutualistic
association of approximately 129 native plant species that are known to be pollinated by 11 different species of hummingbirds in western North America
(Grant 1994). These plants all have flowers that are adapted for hummingbird pollination.
2.2 Pollinator Decline
There is evidence of declines in both managed and wild pollinators. The number of honey bee colonies has been in decline over the past half-century because
of disease, parasites, pesticides, and other factors (National Research Council 2007), and since 2006 beekeepers have experienced record high annual hive
losses of 29 percent or more (Bee Informed Partnership 2014).
Little is known about the status of most of North America’s roughly 4,000 species of native bees. However, the little information that we do have
suggests that many native species are experiencing declines that are similar to or more severe than the declines that we have seen in honey bees. For
example, a recent analysis of North America’s bumble bees (Bombus spp.) conducted by the International Union for the Conservation
of Nature Bumble bee Specialist Group indicates that one-third of North America’s bumble bees have experienced significant declines (Hatfield et
al. 2012). These include several bumble bees that were formerly among our most common species. This analysis is corroborated by many recent studies that
have documented bumble bee declines throughout North America (Colla and Packer 2008; Evans et al. 2008; Grixti et al. 2009; Colla and Ratti 2010; Cameron
et al. 2011; Colla et al. 2012; Koch and Strange 2012; Bartomeus et al. 2013).
The Xerces Society for Invertebrate Conservation publishes red lists of pollinator species that identify endangered, threatened, and other at-risk pollinator
species and their habitats (Xerces Society Red List of Pollinator Species 2007). The Xerces Red List of bees contains 57 species, including 27 species
of yellow-faced bees (Hylaeus species) endemic to the Hawaiian Islands and many other bees that are found only in the western United States.
Butterfly species have also seen declines. NatureServe has assessed all 800 species of butterflies in the United States and has found that 141 (17 percent)
are at risk of extinction (NatureServe 2014). Twenty-six species of butterflies in the United States are listed as threatened or endangered under the
Federal Endangered Species Act (U.S. Fish and Wildlife Service 2014). Although these lists identify the species most at risk of extinction, little data
exists to document a general decline in butterfly species. Most of the butterflies assessed by NatureServe are rare endemics—those species that have a
narrowly limited geographic range or very specific habitat requirements. However, many lepidopterists across the country are reporting that broadly distributed
butterflies are in decline (personal communication, Dr. Jaret Daniels, University of Florida, Dr. John Shuey, Chair of the Lepidopterists’ Society’s
Conservation Committee, Dr. Art Shapiro, University of California at Davis). Emblematic of the decline in wide-ranging butterflies is the precipitous
decline in the monarch butterfly.
There is clear evidence that the monarch butterfly population is declining to dangerously low levels. The best available population size estimate for
monarchs is based on the number of individuals at overwintering sites. Numbers of monarchs that overwinter in Mexico are extrapolated from the area of
overwintering habitat that is occupied by monarchs. The number of monarchs that overwinter in Mexico has been extrapolated from the combined area of overwintering
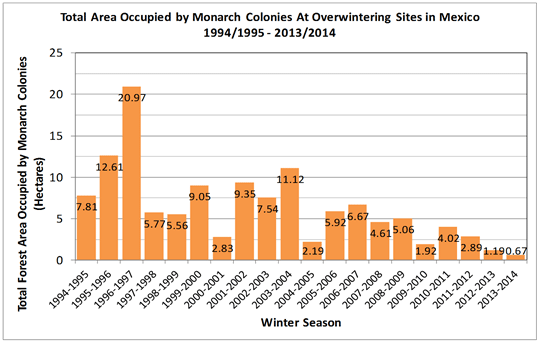
sites (Brower et al. 2012), and assume that approximately 50 million monarchs occur per hectare (Slayback et al. 2007). In the 1990s, hundreds of millions
of monarchs made the epic flight each fall from the northern plains of the United States and Canada to sites in the oyamel fir (Abies religiosa)
forests north of Mexico City, where they occupied, on average, 9.25 hectares of forests each winter. Monarch butterfly numbers have been declining for
more than a decade, and in 2014 scientists observed the lowest numbers ever documented (Rendon-Salinas and Tavera-Alonso 2014). In fact, only 0.67 hectares
(1.65 acres) of forest area occupied by monarch butterflies were observed during winter 2013-2014 at Mexican overwintering sites, which represents a 90
percent decline in monarch butterfly numbers (Rendon-Salinas and Tavera-Alonso 2014) (Figure 1).
Figure 1. Area of Forest Occupied by Colonies of Hibernating Monarchs in Mexico (Graph Courtesy of the Monarch Joint Venture)
In western North America, a smaller population of monarch butterflies makes a shorter annual flight from inland farms, rangelands, and natural areas to
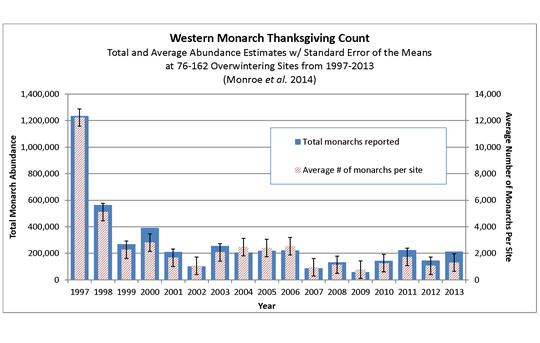
overwintering sites along the California coast. As with their eastern counterparts, significant declines of monarchs have been observed at California
overwintering sites. The western monarch butterfly population (that overwinters in more than 200 groves along the California coast) consists of several
hundred thousand butterflies, on average, although a peak number of 1.2 million butterflies was counted in 1997 (Monroe et al. 2014). Monitoring of the
western population over a 17-year period shows a significant decline in both the total number of butterflies reported per year and the average number
of monarchs per site. Western monarch butterfly population estimates from the fall of 2013 show a 50 percent decline from 1997 (Monroe et al. 2014) (Figure
2).
Figure 2. Monarch Estimates at California Overwintering Sites from 1997 to 2013
A recently completed assessment funded by the U.S. Forest Service and undertaken by NatureServe and the Xerces Society for Invertebrate Conservation has
found that monarch butterflies in North America are vulnerable to extinction (Jepsen et al 2015).
The authors used NatureServe’s conservation status assessment methodology to determine the level of imperilment of the monarch. Using data on population
abundance, trends, and threats, the team of scientists determined that while the monarch butterfly species as a whole, Danaus plexippus,
is apparently secure, the subspecies occurring in North America, Danaus plexippus plexippus, is vulnerable to extinction. Under the assessment,
the North American monarchs were split into an eastern population that migrates from as far north as southern Canada to central Mexico each fall, and
a smaller western population, that largely migrates to coastal California to spend the winter. The eastern monarch population was assessed as “critically
imperiled” due to recent rapid decline and widespread threats. The western population, with a slightly slower rate of decline and less widespread
threats, was categorized as “vulnerable to imperiled.”
Three factors appear most important to explain the decline of eastern monarchs: loss of milkweed breeding habitat due to increased use of herbicides on
genetically modified herbicide-resistant cropland and land conversion, logging at overwintering sites, and climate change and extreme weather (Jepsen
et al 2015). In addition, natural enemies such as diseases, predators, and parasites, as well as pesticides used in agricultural areas may also contribute
to the decline (Jepsen et al 2015). The loss of milkweeds, the monarch’s required larval host plants, has been significant, particularly within
agricultural fields (Pleasants and Oberhauser 2012; Hartzler 2010).
At the XX Annual Meeting of the Canada/Mexico/U.S. Trilateral Committee for Wildlife and Ecosystem Conservation held in April 2015 in San Diego; Canada,
the United States and Mexico all made a commitment to work together to conserve habitat in all three countries.
The populations of both hummingbirds and nectar-feeding bats throughout the southwestern United States have experienced declines due to a variety of pressures
including the disruption of migratory routes and loss of habitat (National Research Council 2007). Hummingbirds in the Southwest face pressures from development
of coastal and desert scrub habitat, and the invasion of nonnative grasses and the loss of stopover habitat used during their long migrations (Calder
2004 ).
North American nectar-feeding bats, such as the lesser long-nosed bat and Mexican long-nosed bat, are believed to be undergoing long-term declines in
their populations and are both federally listed as endangered due to disturbance of their roost sites and removal of foraging habitat and nectar sources
(U.S. Fish and Wildlife Service 2006 ). The listing for these species was triggered by the loss of habitat for maternity colonies as well as the reduction
in acreage of one of their primary sources of nectar, agave, which has been increasingly harvested for the manufacture of mescal and tequila (Arizona
Game and Fish Department 2003 ). Only a limited number of caves and mines are known that provide the necessary environment for these bats (Richardson
2005 ). Many of these sites are facing disturbance pressures within both North America and Mexico, covering the majority of their range (Arizona Game
and Fish Department 2003 ). Roost sites face disturbance pressures from activities such as recreational caving, mine closures, and development. Many caves
in North America are now protected, though the ability to enforce and protect their habitat within Mexico remains uncertain (Hutson et al. 2001).
2.3 Benefits of Roadsides to Pollinators
Concerns about declining pollinator populations in North America (National Research Council 2007; Cameron et al. 2011; Brower et al. 2012a) have increased
interest in investigating and promoting habitat that can support pollinators (National Research Council 2007). In substantially altered environments,
marginal habitat such as roadsides, power line ROWs, hedgerows, and field edges may be especially important for the conservation of biodiversity. Typically
dominated by early-successional plant communities with intermittent disturbance, these areas can support a high diversity of pollinators (e.g., Russell
et al. 2005; Haaland and Gillin 2010; Morandin and Kremen 2013b), including imperiled or federally listed endangered species (e.g., Forrester et al. 2005).
An immense amount of land is dedicated to roadsides, which form one of the most extensive networks of linear habitats on earth. In the United States,
roadsides managed by State DOTs cover more than 10 million acres of land. Roadsides provide an even higher percentage of potential habitat in highly developed
areas, including nearly 1 percent of land in Great Britain (Way 1977 ) and between 1.5 and 2.1 percent of land in the Netherlands (Huijser and Clevenger
2006 ). Landscape coverage by roadsides in Sweden is nearly equal to the amount of grasslands (Gerell 1997 ). Remnant or seminatural habitat, habitat
that can be found on roadsides, is linked to the maintenance of healthy ecosystems and provision of ecological services, including crop pollination services
(Kremen et al. 2002b ; Garibaldi et al. 2013 ).
Roadsides can provide habitat for pollinators, offering forage for food, breeding, or nesting opportunities, and can also aid dispersal of pollinators
by linking fragmented habitats.
Though surveys of pollinators on roadsides are limited, existing studies demonstrate that pollinators can be numerous in roadsides. Nearly 800 bumble
bees were netted from flowers on four Iowa roadsides, suggesting that sizable populations of bumble bees forage on roadside vegetation (Hopwood et al.
2010). Researchers in Finland marked 2,113 ringlet butterflies in a single large intersection and, based on their recaptures of marked butterflies, estimated
a total population of 9,399 butterflies (Valtonen and Saarinen 2005). In a larger survey of 51 roadsides in Finland, 5,964 individual butterflies and
4,626 moths were collected (Saarinen et al. 2005).
Pollinator diversity can also be high in roadsides. Ries et al. (2001 ) observed 42 percent of the butterfly species found in Iowa on roadsides. In Kansas,
Hopwood (2008 ) found that roadsides with native prairie vegetation had bee diversity similar to that of a prairie remnant. In a small-scale survey of
Dutch roadsides, researchers found 63 species of bees (19 percent of Dutch bee fauna), 61 species of hoverflies (18 percent of fauna), and 25 species
of butterflies (35 percent of fauna) (Noordijk et al. 2009). A survey along a highway in Britain found four species of butterflies, five species of bees,
five species of beetles, and 24 species of syrphid flies, all collected within one 200-meter stretch of roadside (Free et al. 1975). Another survey of
12 roadsides in Britain found 27 butterfly species, 47 percent of the species known to Britain (Munguira and Thomas 1992 ). Roadsides can be home to rare
species of butterflies as well as common species (Munguira and Thomas 1992 ; Ries et al. 2001 ).
2.3.1 Ecological Functions Provided by Roadsides
Food
Pollinators such as beetles, flies, wasps, moths, butterflies, bees, hummingbirds, and bats all forage for food on flowers. Nectar and pollen are sources
of carbohydrates and protein, respectively, and pollination is an incidental effect of feeding or gathering food. Flowering plants in roadsides are important
sources of nectar and pollen for pollinators that reside within the roadside habitat (e.g., Munguira and Thomas 1992) as well as those that use the roadside
as a partial habitat and reproduce or overwinter elsewhere (e.g., Ouin et al. 2004). Adult butterflies, syrphid flies, wasps, bees, hummingbirds, bats,
and some moths and beetles feed on nectar to maintain their energy levels. Some adult beetles and syrphids require the protein that pollen provides in
order to reproduce. Female bees actively collect pollen to take back to their nests, where they provide for their offspring by leaving a supply of pollen
moistened with nectar.
Pollinator insects all have complex life cycles, with certain resources needed during different life stages. Roadsides can provide resources needed for
one life stage (e.g., nectar plants for adult butterflies) but also have the potential to provide resources needed for all life stages (e.g., host plants,
nectar plants, and overwintering habitat for butterflies). The availability of floral resources influences the abundance and diversity of butterflies
(Saarinen et al. 2005) and bees (Hopwood 2008) found on roadsides.
Roadsides can wholly support butterfly and moth populations if nectar and host plants are sufficiently abundant (Munguira and Thomas 1992; Saarinen et
al. 2005). Although host plants for monarchs are in decline in many landscapes, roadsides remain an important source of milkweeds for monarchs; approximately
10 percent of remaining milkweeds grow in roadsides (Flockhart et al. 2014 ). Even butterflies that require a narrow range of host plants, such as the
Karner Blue butterfly (Lycaeides melissa samuelis), whose caterpillars will only survive on lupine (Lupinus perennis), can breed on
roadside habitat (Forman et al. 2003; Smallidge et al. 1996). Roadsides have been identified as breeding habitat for at least 25 of the 60 species of
butterflies found in Great Britain (Way 1977).
Shelter and Nest Sites
Pollinators have additional habitat needs, such as shelter, sites for nesting or egg-laying, or overwintering habitat that roadsides can also provide.
Bees provide for their young by constructing nests in which their offspring develop. Many ground-nesting bees prefer to nest in sunny, bare patches of
soil (Linsley 1958). Such patches can be found around the bases of native bunch grasses such as little bluestem (Schizachyrium scoparium) that
tend to grow in dense bundles, leaving small areas of bare ground exposed between plants. Hopwood (2008) found that ground-nesting bees in Kansas were
more common in roadsides with native plantings. In contrast, roadsides with a tight sod of brome or other nonnative cool season grasses had fewer ground-nesting
bees. Researchers in the Netherlands found 11 species of bees nesting in the ground in roadside areas (Schaffers et al. 2012 ).
Roadside vegetation can also provide habitat for tunnel-nesting bees, which nest in hollow or pithy stems or other small cavities. Bumble bees require
a small, insulated cavity, such as underneath grass clumps (Svennson et al. 2000) or under the thatch of bunch grasses (Hatfield et al. 2012). Eight of
the 17 species of bumble bees found in Great Britain have been recorded nesting in roadsides (Way 1977).
The breeding and overwintering habitat needs are less understood for other groups of pollinators, but Schaffers et al. (2012 ) has recorded syrphid fly
species and soldier beetles overwintering in roadside soil or litter. Butterflies and moths may also utilize roadsides as overwintering habitat (Schaffers
et al. 2012 ) or shelter (Saarinen et al. 2005).
Landscape Connectivity
Landscape connectivity is important for the populations of many species, but due to urbanization, agricultural intensification, and other human activities,
habitat is becoming increasingly fragmented (Saunders et al. 1991). Roadsides extend across a variety of landscapes, often contain greater plant diversity
than adjacent lands, and are generally excluded from further development and major disturbances. In developed landscapes, such as intensively managed
agricultural lands, roadsides may be refugia for pollinators in an otherwise inhospitable environment. The linear shape and connectivity of roadsides
may help pollinators move through the landscape, either for daily foraging or for dispersal between larger habitat patches. Additionally, roadside habitat
corridors are likely to be particularly beneficial in agricultural landscapes, where natural or seminatural habitat benefits pollinator populations (Menz
et al. 2011; Klein et al. 2012; Morandin and Kremen 2013b) as well as crop yields (Morandin and Winston 2006; Blaauw and Isaacs 2014; Klatt et al. 2014).
Corridors, strips of habitat that connect larger patches of habitat, have the potential to facilitate movement of organisms between habitat fragments,
aid in establishing or maintaining populations, and increase species diversity within isolated areas (Tewksbury et al. 2002). Corridors may not always
need to directly connect habitat areas to help organisms to disperse (Fried et al. 2005); patches of habitat, such as native plants or even individual
trees, can serve as stepping stones between isolated fragments in otherwise inhospitable landscapes (Ottewell et al. 2009).
While populations of some pollinators may not fully be supported by roadside habitat, roadsides may direct their movement to other, more complete patches
of habitat. Different pollinator guilds have differing dispersal capabilities, which will affect their ability to use roadsides as corridors. Wild bees,
for example, disperse into agricultural field margins when close to remnant habitat but their abundance in margins declines with distance from remnant
habitat (Jauker et al. 2009 ). Pollinators with limited dispersal capability, such as tiny sweat bees that have foraging ranges of less than 200 meters
(Greenleaf et al. 2007 ; Zurbuchen et al. 2010 ), may need roadsides that connect directly to habitat to aid their dispersal. In contrast, bumble bees,
which can fly up to 2 kilometers (km ) or more (Osborne et al. 2008 ), and other larger-bodied pollinators may be able to colonize new habitat patches
using noncontiguous roadsides as stepping stones.
Although no studies have specifically tested if roadsides can link fragmented pollinator habitat, there is evidence to suggest that pollinators use roadsides
as corridors, whether to move through the landscape in search of food or in pursuit of new habitat. Experimental corridors have been found to increase
the movement of pollinators (Haddad 1999; Haddad and Baum 1999) as well as facilitate pollination (Tewksbury et al. 2002; Townsend and Levey 2005). Recent
research indicates that existing linear habitats, such as field borders, hedgerows, avenues, and green lanes, can act as corridors for pollinators (Sutcliffe
and Thomas 1996; Croxton et al. 2005; Van Geert et al. 2010; Lentini et al. 2012; but see Ockinger and Smith 2008; Krewenka et al. 2011). Using experimental
strips designed to simulate roadsides in Sweden, Soderstrom and Hedblom (2007) observed that specialist butterflies released nearby moved within the strips
to disperse.
Ries et al. (2001 ) found habitat-sensitive butterflies in Iowa were more likely to stay and move within roadsides with prairie vegetation than roadsides
with weedy vegetation. Hopwood et al. (2010) found that bumble bees moved up to 900 meters (m) within Iowa roadsides. Asphalt roads direct the flight
of syrphid flies along roadsides rather than across roads (Lovei et al. 1998). In Finland, ringlet butterflies (Aphantopus hyperantus) also moved
within roadsides, and moved more frequently between nearby habitat patches that were connected with roadsides (Valtonen and Saarinen 2005). There is also
data to indicate that more species and more individuals of habitat-sensitive butterflies were found in roadsides planted with native vegetation than surrounding
land, suggesting that roadsides could serve as corridors for some butterflies (Ries et al. 2001). Perhaps most compelling is the evidence of range expansions
along roadsides with host plants reported for the Cinnabar moth (Tyria jacobaeae) in Germany (Brunzel et al. 2004 ) and the Silvery Blue butterfly
(Glaucopysche lygdamus couperi) in Canada (Dirig and Cryan 1991 ). Although we have some evidence for pollinators using roadsides as movement
corridors, additional research examining how much pollinators use roadsides as corridors, and whether roadsides reduce isolation of pollinator populations,
is needed.
Back to top
Chapter 3: Threats to Pollinators Associated with Roads and Roadsides
Globally, pollinators are in decline (National Research Council 2007; Biesmeijer et al. 2006; Potts et al. 2010). Threats such as the loss, degradation, and fragmentation of habitat (Kremen et al. 2002b; Williams et al. 2007; Potts et al. 2010); introduced species (Memmott and Wasser 2002; Tallamy and Shropshire 2009; Hanula and Horn 2011; Fiedler et al. 2012); the use of pesticides (Kevan 1975, 1999; Dover et al. 1990; Kearns and Inouye 1997; Alston and Tepedino 2000; Whitehorn et al. 2012; Desneux et al. 2007; Baron et al. 2014); habitat disruption from grazing, mowing, fire, and indirect effects of biological weed control (Hatfield and LeBuhn 2007; Johst et al. 2006; Potts et al. 2005; Louda et al. 1997); and diseases and parasites (Altizer and Oberhauser 1999; Altizer et al. 2000; Thorp et al. 2003; Colla et al. 2006; Evans et al. 2008; Williams et al. 2007; Cameron et al. 2011; Koch and Strange 2012) all contribute to pollinator decline.
The loss of pollinators negatively affects plant reproduction and plant community diversity (Bawa 1990; Fontaine et al. 2005; Brosi and Briggs 2013). Threats to pollinators may have profound consequences for ecosystem health as well as our food systems (Kearns et al. 1998; Spira 2001; Steffan-Dewenter and Westphal 2008). Concerns about pollinator decline and its repercussions have led to increased efforts to reduce threats to pollinators. Managing existing habitat for pollinators and restoring additional habitat has been demonstrated to increase pollinator abundance and diversity (e.g., Fiedler et al. 2012; Klein et al. 2012; Morandin and Kremen 2013b), and roadsides are a conservation opportunity to increase pollinator habitat.
However, there are also threats to pollinators specifically associated with roads. Roadside vegetation management can be harmful to pollinators (e.g., Johst et al. 2006). Roads can be a source of mortality for pollinators due to collisions with vehicles (e.g., Munguira and Thomas 1992). Roads fragment and degrade habitat (Trombulak and Frissell 2000). Roads may act as barriers to pollinator movement (e.g., Valtonen and Saarinen 2005). The prevalence of invasive and nonnative species on roadsides reduces pollinator abundance and diversity (e.g., Hopwood 2008). Finally, roadsides are exposed to drift from adjacent land (e.g., Krupke et al. 2012) and to pollution from vehicles (e.g., Jablonski et al. 1995).
3.1 Roadside Vegetation Management
Mowing and herbicide use are the most commonly used roadside vegetation management tools (Harper-Lore et al. 2013). Grazing and prescribed fire may also be used to manage roadside vegetation. Fire can rejuvenate roadsides planted with prairie species (Schramm 1990), while grazing can be used to target certain noxious weeds (Harper-Lore et al. 2013). These management techniques can be damaging to pollinators under some circumstances, and consideration of pollinators is needed when these tools are used on roadsides. (See Chapter 5 for more information.)
3.1.1 Mowing
Mowing is frequently used to maintain roadside vegetation, reducing invasive weeds and encroaching woody plants. Mowing can have a significant impact on pollinating insects through direct mortality, particularly for egg and larval stages that cannot avoid the mower (Di Giulio et al. 2001). Mortality due to mowing when eggs and larvae are present is a threat to the persistence of some butterfly species (Thomas 1984; Wynhoff 1998). Mowing can also disturb ant nests, which in turn affects the survival of butterflies that rely on particular ant species (their final instar larvae feed in the ant nests) (Wynhoff et al. 2011). Caterpillars on the ground as well as caterpillars on vegetation are vulnerable to direct mortality by mower (Humbert et al. 2010).
Mowing also creates a sward of uniform height and may destroy topographical features such as grass tussocks (Morris 2000) when care is not taken to avoid these features or the mower height is too low. Such features provide structural diversity to the habitat and offer potential nesting sites for pollinator insects such as bumble bees. In addition to direct mortality and structural changes, mowing can result in a sudden removal of almost all floral resources for foraging pollinators and butterfly host plants (Johst et al. 2006). The reduction in host plants and foraging resources can reduce pollinator reproduction and survivorship (Boggs and Freeman 2005), and pollinators will likely be forced to seek alternative habitat. Skórka et al. (2013) found that butterfly roadkill in Poland increased as mowing frequency increased; adult butterflies that dispersed to find new habitat after roadsides were mowed were more likely to collide with vehicles.
The frequency and timing of mowing influences the composition of vegetation over time (Forrester et al. 2005), thus indirectly influencing pollinator diversity and abundance. Frequent mowing during a growing season reduces native plant growth and the ability of forbs to compete with grasses. Excessive roadside mowing may have led to a decrease in flowers and a subsequent decrease in bumble bees in Belgium (Rasmont et al. 2006). Intensively mowed ROWs generally have the shortest vegetation and lowest amount of nectar, which together result in decreased butterfly abundance (Gerell 1997; Saarinen et al. 2005).
However, carefully timed roadside mowing can have positive effects on plant diversity (Parr and Way 1988) that in turn benefit pollinators (e.g., Noordijk et al. 2009). Additional information on effects of mowing frequency and timing can be found in Chapter 5.
3.1.2 Herbicides
Herbicides can be a valuable management tool to control woody vegetation as well as invasive weed species on roadsides. However, herbicide use has both indirect and direct effects on pollinators.
Herbicide use on roadsides can change the composition of the plant community (Tyser et al. 1998), killing the plants that pollinators depend on. Herbicides remove plants that are sources of pollen and nectar for pollinators (Kearns et al. 1998). Nesting habitat or plant materials used by bees in nesting are reduced by herbicide use (Kearns et al. 1998). Similarly, butterfly host plants are destroyed by herbicides (Smallidge and Leopold 1997), as are plants on which larval beetles and flies find alternate food sources.
A pollinator community requires consistent sources of nectar, pollen, host plants, and nesting material during times adults are active, and a reduction in resources can cause a decline in pollinator reproductive success, survival rates, and eventually populations (Kevan 1999; Boggs and Freeman 2005). The disappearance of roadside plants beneficial to pollinators may force pollinators to emigrate to find habitat elsewhere. Pollinators that are forced to leave roadsides with low plant diversity to find more suitable habitat have an increased risk of mortality by passing vehicles (Skórka et al. 2013).
While the majority of the effects herbicides have on pollinators is mediated through changes in vegetation, there is evidence that some herbicides such as paraquat, the organic arsenicals, and phenoxy materials can have lethal effects in bees, either through direct application or exposure by feeding (Johansen and Mayer 1990). 2,4-D and sethoxydim are directly toxic to honey bees (Mader et al. 2010).
Ingestion of herbicides by other insects, such as beetle and butterfly species, has varying effects depending on the species, life stage of the species, and the chemical (Brown 1987; Kegel 1989; Kjaer and Elmegaard 1996; Kjaer and Heimbach 2001; Kutlesa and Caveney 2001; Russell and Schultz 2010; LaBar and Schultz 2012). For example, sethoxydim and fluazifop-p-butyl herbicides both reduce development time of Puget blue butterflies (Plebejus icarioides blackmorei), but reduce survival, pupal weight, and wing size of cabbage white butterflies (Russell and Schultz 2010). Similarly, three commonly used herbicides (triclopyr, sethoxydim, and imazapyr) reduce survivorship of Behr's Metalmark butterflies (Apodemia virgulti) (Stark et al. 2012). Glufosinate-ammonium is highly toxic to larvae of the Brazilian skipper (Calpodes ethlius) (Kutlesa and Caveney 2001). A reduction in caterpillars or pupa surviving to adults due to herbicide exposure can affect butterfly populations (Starket al. 2012).
3.1.3 Grazing
Grazing can be a valuable tool for roadside management by limiting shrub and tree succession, providing structural diversity, and encouraging the growth of nectar-rich plants. However, livestock grazing can greatly alter the structure, diversity, and growth habits of the vegetation community, which in turn can affect the associated insect community (Kruess and Tscharntke 2002a). Grazing is usually only beneficial to pollinators at low to moderate levels and when the site is grazed for a short period followed by ample recovery time—and when it has been planned to suit the local site conditions.
Grazing during periods when floral resources are already scarce may result in insufficient forage available for pollinators such as bumble bees, which can cause a decrease in the populations (Carvell 2002; Hatfield and LeBuhn 2007). Grazing can also affect bees by destroying potential nest sites or existing nests and contents, through the direct trampling of adult bees and removal of food resources (Sugden 1985). Studies of livestock grazing on bees also suggest that increased intensity of livestock grazing negatively affects the species richness of bees (Morris 1967; Sugden 1985; Carvell 2002; Vulliamy et al. 2006).
Livestock grazing can adversely affect butterfly populations directly by trampling during immobile life stages (egg, larvae, pupae) or during cool temperatures when adult movement is restricted (Warren 1993). Grazing can also be detrimental to butterfly populations indirectly by altering plant community composition (Stoner and Joern 2004) and stripping habitat of vegetation, removing adult nectar resources, introducing invasive weeds (Smallidge and Leopold 1997), and changing meadow hydrology (Belsky et al. 1999). As grazing intensity increases, numbers of butterflies and other pollinators decrease (Dana 1997; Balmer and Erhardt 2000; Kruess and Tscharntke 2002a, 2002b). However, light rotational grazing can maintain vegetation heights and habitat heterogeneity favorable to some butterflies (Ravenscroft 1994; Thomas and Jones 1993; Davies et al. 2005) and can increase nesting opportunities for ground-nesting bees (Vulliamy et al. 2006).
3.1.4 Fire
Prescribed fire is a tool used to manage roadside vegetation in some regions with a history of natural fires. Prescribed burns can benefit vegetation and, if used appropriately, fire benefits many insect communities through the restoration and maintenance of suitable habitat (Huntzinger 2003; Hartley et al. 2007; Campbell et al. 2007). However, burns can also be detrimental to pollinator populations (e.g., Ne'eman et al. 2000; Panzer 2002).
Burns during the growing season remove floral resources, host plants, and nesting materials, and can be detrimental to species with life stages that cannot fly to safety at the time of the burn. Burns during the dormant season can kill overwintering pollinators such as butterflies, moths, syrphid flies, and soldier beetles that overwinter at the base of plants, in leaf litter, or underneath the surface of the soil. A recent study on prescribed burning and the imperiled mardon skipper in California showed substantially fewer butterflies in the burned areas of meadows compared to unburned areas after 1, 2, 3, and 5 years following the burn event (Black et al. 2014). Queen bumble bees overwintering in small cavities just below or on the ground surface are at risk, as are a small number of ground-nesting bee species that nest in shallow burrows (Cane and Neff 2011). Solitary bees nesting in stems or twigs are unlikely to survive the heat of burns (Cane and Neff 2011), and stem-nesting bee populations will only recover post fire when the availability of suitable stems increases over time (Potts et al. 2005). The loss of bees due to a burn can lead to reduced fruit sets in plants in burned areas (Ne'eman et al. 2000). Though losses of bees following a fire can be catastrophic, bees may be able to recolonize burned sites and recover within a few years (Potts et al. 2003a).
Recovery of pollinators following a burn varies between guilds. Habitat-dependent or -specialist species and those that are less mobile are most likely to be negatively affected immediately by a fire (Panzer 2002; Vogel et al. 2010). A pollinator's ability to cope with regular burns is dependent on there being adequate unburned adjacent areas that can provide sources of colonizers into the burned habitat (e.g., Harper et al. 2000; Swengel 2001; Panzer 2002; Hartley et al. 2007). Isolated populations of pollinators in small fragments may not survive repeated prescribed burns (Panzer 2002) because there are often no source populations available for recolonization once a population has been locally extirpated. Burning a small habitat fragment in its entirety could risk extirpating some species because of limited recolonization from adjacent habitat (Harper et al. 2000). This accentuates the need to leave substantial habitat when using fire as a management tool. Habitat patches should not be burned completely; rather, a mosaic of burned and unburned areas is ideal.
3.1.5 Biological Control
To control introduced, invasive species of weeds that cause significant economic damage, natural enemies such as insects or pathogens may be introduced from the region of the weed's origin. This process of introducing natural enemies of introduced pest species is known as classical biological control. Currently, biological control of weeds is not widely implemented by State DOTs, but several have released natural enemies to control weeds such as purple loosestrife (Lythrum salicaria), leafy spurge (Euphorbia esula) (Johnson 2000), yellow star thistle (Centaurea solstitialis), and Russian thistle (Salsola kali) (Harper-Lore et al. 2013).
There are several steps involved in classical biological control, including identifying effective natural enemies of the target pest; testing the natural enemy in quarantine; and, finally, establishing permanent populations of the nonnative biocontrol agent at a regional scale. Introductions are regulated by the U.S. Department of Agriculture and are monitored by government scientists, university researchers, and State agencies.
Biological control can be an effective focused approach to weed control. However, there are ecological and economic risks associated with introducing a species outside of its natural range. Forecasting the broader ecological effects of an introduced species on other non-target species is very difficult and there is always a potential for unpredictable and irreversible consequences (Simberloff and Stiling 1996).
Risks are highest to plants, and the species that depend on those plants, that are closely related to target weeds (Pemberton 2000). For example, the Eurasian weevil (Rhinocyllus conicus) was introduced to North America in 1969 to control exotic thistle species in the genus Carduus. Less than 10 years later it was detected feeding on flower heads of native thistles (Reese 1977), and eventually expanded its host range to three genera of native thistles (Louda et al. 1997). The beetle also moved beyond the initial release sites, and can now be found in over 25 States (Louda et al. 1997). The weevil can cause substantial harm to native thistles by significantly reducing seed production and may be a threat to rare thistle species (Louda et al. 1997; Louda and O'Brien 2002). Native picture winged flies that feed on native thistles declined as beetle density increased (Louda et al. 1997). Butterflies, moths, bees, wasps, beetles, and flies all visit native thistles; one species of bee, Melissodes desponsa, specializes in the pollen from Cirsium species. Pollinators that rely on native plants for pollen and nectar will be harmed by reductions in abundance due to herbivorous insects introduced to control weeds.
The possibility of host switching, and the difficulty of predicting ecological interactions when introducing a species, are serious limitations of classical biological control. Species introduced as biological control agents can have a negative impact on pollinators and disrupt native ecosystems, and the introductions of species and their resulting interactions cannot be reversed.
3.2 Mortality Due to Traffic
Collisions with passing vehicles can be a source of mortality for many animals, including pollinators (Pickles 1942; Seibert and Conover 1991). Further quantifying the impacts of vehicular traffic on pollinators is challenging; studies of the impacts of traffic on insects are few.
The height at which pollinators may cross roads is not well known, although some pollinators fly at heights that would be in the path of vehicles. One observation from Severns (2008) suggests that Fender's blue butterflies tend to cross the road at heights of less than a meter. Vinchesi (2014) reports that alfalfa leafcutter bees (Megachile rotundata) and alkali bees (Nomia melanderi) tend to cross roads at heights of 2 meters or less. Rao and Girish (2007) note that butterfly species that fly low or congregate when mud-puddling near the side of the road are more prone to fatalities than those species that fly above vehicles. Migrating monarchs that fly low to the ground in windy weather or at certain times of the day may be more susceptible (McKenna et al. 2001).
Butterflies appear to be one of the more common groups of insects to be killed along roads (Rao and Girish 2007). McKenna et al. (2001) estimate that hundreds of thousands of butterflies are killed weekly in the state of Illinois. Munguira and Thomas (1992) found that mortality rates of butterfly species found on roadsides in Britain due to vehicles were between 0.6 percent and 7 percent, but the authors considered the mortality rates to be small compared with mortality due to natural factors. About 10 percent of observed Oregon silverspot butterflies, a threatened species, were killed by collisions with vehicles (Zielin et al. 2010). In roadside surveys of butterflies in Poland, Skórka et al. (2013) found that some species were more likely to be killed by vehicles than others, but on average about 8.2 percent of individuals for a particular species were killed on roads. McKenna et al. (2001) and Rao and Girish (2007) found greater mortality of male butterflies. In the case of monarch butterflies, McKenna et al. (2001) suggest it may be because of their penchant for chasing other butterflies.
Surprisingly, a roadside inventory of dead butterflies and moths in Illinois found that observed mortality was highest at an intermediate level of traffic, with lowest mortality at the highest and lowest rates of traffic (McKenna et al. 2001). Additional studies support that the amount of traffic on adjacent roads does not appear to influence the numbers of butterflies (Munguira and Thomas 1992; Thomas et al. 2002) or bee richness or abundance (Hopwood 2008) in roadside habitats. In Iowa, research found that more butterflies were killed in predominantly grassy roadsides than in roadsides planted with prairie vegetation, and only 2.8 percent of butterflies observed crossing the road were hit by cars (Ries et al. 2001). Saarinen et al. (2005) studied butterfly and moth communities along Finnish roads varying in road size and traffic volumes and found that diversity and abundance of butterflies and moths were not affected by traffic volume. However, Skorka et al. (2013) and Rao and Girish (2007) both report increased butterfly mortality due to increased traffic volume. Road width also increased mortality (Skorka et al. 2013).
Skórka et al. (2013) found that the frequency of mowing was linked to the proportion of butterflies killed on roads; butterflies that had to disperse to find new habitat after roadsides were mowed may have had a greater likelihood of collisions with vehicles. The researchers also found that roadsides with more species of plants had fewer butterflies killed by traffic, and that wider roadsides also decreased traffic mortality (Skórka et al. 2013). If quality roadside habitat is present, it may reduce the amount of pollinators killed by vehicles by providing pollinators with necessary habitat and less need to disperse elsewhere.
Though limited to a few instances, mitigation efforts to reduce insect deaths during road crossings include speed reduction signs (Zielin et al. 2010). In Washington, where there is a high density of nesting beds of the alkali bee, an important pollinator of alfalfa, speed limits are imposed on county roads and highways during bee foraging season to reduce mortality (Vinchesi 2014).
Based on the information presented above we believe the preponderance of evidence suggests that the benefit to pollinators from the management of suitable native habitat on roadsides outweighs the risks to pollinators from potential impacts associated with passing vehicles.
3.3 Roads and Habitat Modification
Roads can modify habitat significantly, with consequences for pollinators. Roads fragment once-continuous habitat and may be barriers to pollinator movement. Roadsides are subjected to invasive species, pollutants, and insecticide drift from adjacent land.
3.3.1 Roads and Habitat Fragmentation
Roads can contribute to habitat loss and fragmentation (Spellerberg 1998; Forman et al. 2003). Construction of roads removes habitat and can damage or degrade remaining land (Coffin 2007). Roads also dissect large areas into smaller patches, isolating populations (Trombulak and Frissell 2000).
Habitat loss, degradation, and fragmentation are linked to declines in pollinator diversity and abundance (Frankie et al. 1990; Allen-Wardell et al. 1998), changes in pollinator community composition (Brosi et al. 2008; Ricketts et al. 2008; Krauss et al. 2009; Winfree et al. 2009) that is followed by a reduction in pollination services (Kremen et al. 2002a), and decreased population sizes or densities of individual pollinator species (Kearns et al. 1998; Spira 2001). If habitat becomes fragmented and the distance between patches is greater than the foraging range of pollinators, patches may be too small to support their own pollinator fauna and may be isolated from other habitat where pollinators are present (Goverde et al. 2002; Kearns et al. 1998; Osborne and Williams 2001; Williams and Kremen 2007).
3.3.2 Roads as Potential Barriers to Pollinator Movement
Movement is fundamental to a pollinator's life, and roads have the potential to be barriers, dividing and blocking movement between habitats. Although literature describing possible barrier effects of roads on pollinators is limited, the degree to which roads are restrictive to pollinators appears to vary between species and food availability.
Roads may restrict the movement of some butterflies (Fry and Robson 1994). However, different species of butterflies with different life history characteristics respond to roads differently (Ries and Debinski 2001). A habitat-specialist butterfly is less likely to cross a road edge than a migrating butterfly (Ries and Debinski 2001). The width of road or overall density of roads in the landscape may influence butterfly response to roads. Although a single road is not a barrier for ringlet butterflies (Aphantopus hyperantus), a dense network of roads can decrease movement (Valtonen and Saarinen 2005).
There is also evidence to suggest that roads are not barriers to butterflies. In a thorough study of butterfly diversity, mortality, and movement within roadsides, Munguira and Thomas (1992) concluded that roads could not be considered barriers to the movement of any butterflies they observed. Roads are also not thought to be a barrier to the dispersal of the endangered Fender's blue butterfly (Icarica Icarioides fenderi) in Oregon (Severns 2008).
Studies of movement across roads by other pollinators are limited. Hover flies in an agricultural landscape preferred to avoid crossing asphalt roads and other barriers, such as hedgerows (Lövei et al. 1998). Bees, which are extremely adept fliers, are able to cross roads (Bhattacharya et al. 2003; Hopwood et al. 2010; Vinchesi 2014). However, bumble bees appear reluctant to cross roads unless they need to seek floral resources elsewhere (Bhattacharya et al. 2003; Hopwood et al. 2010). It is difficult to know without further investigation whether roads are actual barriers to bumble bee movement or if the observed tendency to return to their preferred floral patch on one side of the road is due to high site fidelity, a behavior described by Dramstad (1996) and Osborne and Williams (2001).
3.3.3 Introduced Plant Species
Many nonnative and invasive plants are disproportionately present in many roadsides (Tyser and Worley 1992; Gelbard and Belnap 2003). Roadsides often have optimal conditions for plant introductions and invasions, with abundant light, nutrients from adjacent land, and reduced competition from trees and shrubs for water (Christen and Matlack 2009). Nonnative species are dispersed as propagules such as seeds and spread by vehicles (Von der Lippe and Kowarik 2007). Nonnative plants have also been planted intentionally as part of roadside vegetation management efforts (Rentch et al. 2005). Additionally, roadsides extend uninterrupted for miles, providing dispersal corridors for nonnative plants (Hansen and Clevenger 2005).
Nonnative plants can decrease the quality of roadside habitat for pollinators (Hopwood 2008; Valtonen et al. 2006). Introduced nonnative plants compete with native plants for resources as well as alter habitat composition, and some cause significant reductions in the abundance and diversity of pollinators and other herbivorous insects (Samways et al. 1996; Kearns et al. 1998; Spira 2001; Memmott and Wasser 2002; Hopwood 2008; Zuefle et al. 2008; Burghardt et al. 2009; Tallamy and Shropshire 2009; Wu et al. 2009; Hanula and Horn 2011; Fiedler et al. 2012). There is also evidence that native pollinator insects prefer native plants (Hopwood 2008; Burghardt et al. 2009; Wu et al. 2009; Williams et al. 2011; Morandin and Kremen 2013a), even though many native insects will feed on nonnative plants when few natives are available (Zuefle et al. 2008; Burghardt et al. 2009; Wu et al. 2009; Williams et al. 2011).
3.3.4 Roadside Contamination
The use of roads by vehicles and the maintenance of roads contaminate roadsides with pollutants, including heavy metals from gasoline additives, de-icing materials, and vehicle exhaust. Roadside soils and vegetation can be contaminated with heavy metals such as lead, iron, zinc, copper, cadmium, nickel, and others (Gjessing et al. 1984; Oberts 1986; Araratyan and Zakharyan 1988). Contamination is proportional to vehicular traffic (Leharne et al. 1992). In general, plant and soil contamination is greatest adjacent to the road and decreases with distance from the road (Quarles et al. 1974; Dale and Freedman 1982; Jablonski et al. 1995; Swaileh et al. 2004). Contamination tends to decline within 20 meters but can still be present at high levels up to 200 meters from the road (Spellerberg 1998; Trombulak and Frissell 2000). Pollen and nectar contamination is also greatest nearest to the road (Jablonski et al. 1995). Contamination can be found in soil invertebrates or invertebrates feeding on contaminated plants (Williamson and Evans 1972). Pollinators are exposed to heavy metals through direct deposition on their bodies or through ingestion of contaminated plants, pollen, nectar, or water. Few studies exist examining the effects of pollution of heavy metals on pollinators.
Heavy metals may play a role in butterfly declines in Northern Europe indirectly by weakening host plants (Mulder et al. 2005) or directly when caterpillars ingest contaminated plants (Nieminen et. al 2001). Heavy metals can accumulate in the bodies of honey bees (Perugini et al. 2011), and pollen contaminated with zinc has been demonstrated to reduce reproduction and survival of Osmia rufa (Moroń et al. 2010). Wild bee diversity and abundance decreased in meadow sites as heavy metal concentrations increased (Moroń et al. 2012). However, Szentgyörgyi et al. (2011) found that bumble bee diversity did not correlate with concentrations of heavy metals in soils of meadows.
Ozone, nitrates, and other exhaust gases may also have an impact on roadside vegetation and pollinators. Ozone and nitrates can inhibit floral scent, which reduces a pollinator's ability to detect flowers and in turn may reduce reproductive output of both pollinators and plants (McFrederick et al. 2008). De-icing salts used on roads alter roadside soil chemistry and can damage plants (Bogemans et al. 1989), with probable indirect impacts on pollinators.
There are no current surveys of heavy metal or de-icing salt contamination along United States roadsides. Additionally, very little is known about the impacts of those materials on pollinators. Such information would be helpful in designing mitigation strategies. Until those data are available, maintaining a mown strip of vegetation directly adjacent to the road may help reduce pollinator exposure to these contaminants.
3.3.5 Pesticide Drift from Adjacent Land
Roadsides are narrow, linear habitats subject to impacts of the adjacent road as well as effects of adjacent land, including exposure to insecticidal drift. Insecticides are widely used on agricultural lands, in natural areas, and municipal areas throughout the United States. Drift from applications to agricultural crops, wetlands for mosquito abatement, and treatments to forests or rangelands could expose pollinators using roadside habitat. Any application of insecticides can threaten pollinators, but drift from aerial spraying can be particularly harmful; drift can be dangerous to bees for over a mile from the original spray site (Johansen and Mayer 1990).
Insecticides can kill pollinators outright (Johansen 1977), but sub-lethal doses can also affect their foraging and nesting behaviors, reproductive success, and immune responses (Thompson 2003; Decourtye et al. 2004, 2005; Morandin et al. 2005; Desneux et al. 2007). Sometimes the use of insecticides causes a series of effects to ripple through an ecosystem, and can reduce pollination (Kevan 1975, 1999).
Neonicotinoids, a group of insecticides highly toxic to pollinators and widely used on agricultural crops as well as on horticultural and ornamental plants, are increasingly likely to contaminate marginal habitats such as roadsides. Applied to plants as seed treatments, sprays, soil drenches, or bark applications, neonicotinoids are systemic insecticides; the chemicals can move throughout the whole plant, including into pollen and nectar (see Hopwood et al. 2012 for a review). Even low-level exposures can reduce bee reproductive success (Gill et al. 2012; Whitehorn et al. 2012; Sandrock et al. 2013) and foraging ability (Schneider et al. 2012; Gill and Raine 2014; Tan et al. 2014). Since neonicotinoids are long-lived in plants and soil, residues may be present for several years following an application (Jones et al. 2014), and untreated plants may take up residues of neonicotinoids still present in the soil from previous applications (Bonmatin et al. 2003, 2005).
Roadside vegetation may become contaminated with insecticidal dust that sloughs off neonicotinoid-treated seeds during the planting of nearby agricultural fields. This dust can drift off site and kill pollinators on contact but will also contaminate flowering plants, exposing pollinators to toxic residues long after the insecticides were applied to crops (Krupke et al. 2012).
Public education and communication about maintaining habitat quality of roadsides can be effective strategies (Brandt et al. 2011). When possible, roadside managers could work with roadside-adjacent landowners about strategies to reduce pesticide drift, including weather conditions under which to apply pesticides, maintaining adequate buffer zones, and spray formulations and droplet sizes. Alternatively, landscape design of new plantings might include windbreaks of nonpollinator attractive species that can shield roadside vegetation from pesticide drift.
Back to top
Chapter 4: Restoring Habitat for Pollinators
There is a need to enhance and restore habitat for pollinators (Kearns et al. 1998; Menz et al. 2011). Roadsides represent an opportunity to increase pollinator habitat across landscapes. Although adjusting vegetation management techniques to accommodate pollinator resource needs is a key step toward improving the quality of roadside habitat for pollinators (see Chapter 5), roadside restorations can also significantly improve the value of roadside habitat for pollinators. Enhancing and restoring habitat are two of the most valuable ways to conserve native pollinators (Kremen et al. 2007) and provide resources for honey bees.
Although there are threats to pollinators associated with roads (see Chapter 3), some of these threats can be mitigated by roadside habitat restoration. For example, roadsides with remnant or restored vegetation can lessen the effects of habitat fragmentation by functioning as corridors and connecting larger habitat patches (Forman et al. 2003; Huijser and Clevenger 2006). Additionally, if roadside restorations contain floral resources, pollinators will be less likely to seek habitat elsewhere. Furthermore, the risk of being killed by vehicles is reduced (Ries et al. 2001; Skórka et al. 2013). The removal of invasive species increases pollinator abundance and diversity (Hanula and Horn 2011; Fiedler et al. 2012), and roadside restorations that replace invasive plants with native vegetation improve pollinator habitat.
This section reviews the literature relevant to pollinators that informs applied habitat restoration. For a detailed guide on the general subject of revegetation practices for roadsides, readers are referred to Roadside Revegetation: An Integrated Approach to Establishing Native Plants (Steinfeld et al. 2007).
4.1 Considerations for Pollinator Habitat Restoration
Roadside managers can take a more active role in increasing the number of pollinators and the scope of pollination services they provide by improving the quality of roadside habitat. This can be done through habitat restoration or enhancement that includes the following goals:
- Increase the abundance of pollen, nectar, and host-plant resources with use of a diverse range of plants that flower throughout the growing seasons, and
- Foster a vegetation structure that provides nesting, egg-laying, and overwintering locations.
In roadside contexts, such habitat restoration can take the form of mass wildflower plantings, inter-seeding low-statured pollinator-attractive plants in existing turf grass areas, and establishing flowering shrubs and trees in living snow fences, windbreaks, or slope stabilization efforts.
Understanding the habitat requirements of pollinators can help planners identify specific pollinator habitat goals for restoration plans. For example, protecting or providing nest sites is as important as providing flowers to support populations of native bees (Tscharntke et al. 1998; Cane 2001; Potts et al. 2005). Similarly, caterpillar host plants are necessary for strong butterfly populations (Feber et al. 1996). Ideal roadside habitat for pollinators would have nesting, host-plant, and forage resources in the same habitat patch. Pollinators are able to adapt to landscapes in which nesting and forage resources are separated, but it is important that these two key habitat components are not too far apart (Cane 2001; Westrich 1996). Table 1 lists the habitat requirements of the main pollinator groups as well as restoration actions that add to or enhance the habitat functions of roadsides for these pollinators.
Table 1. General Restoration Goals to Meet Pollinator Habitat Requirements
| Pollinators |
Food |
Shelter |
Restoration Goals |
| Bats |
Nectar, pollen, fruit |
Caves and mine shafts, trees, and various structures including bridges |
|
| Bees |
Bumble bees |
Nectar for adults; nectar and pollen collected as provisions for larvae |
Nest in small cavities, underground in abandoned rodent nests, under clumps of grass, or in hollow trees, bird nests, or walls |
- Increase density and diversity of native flowering plants
- Provide native bunch grasses for bumble bee nesting habitat
- Provide areas with partially vegetated well-drained soil
- Provide living and dead pithy and woody vegetation
|
| Ground-nesting bees |
Nectar for adults; nectar and pollen collected as provisions for larvae |
Nest in bare or partially vegetated, well-drained soil |
| Tunnel-nesting bees |
Nectar for adults; nectar and pollen collected as provisions for larvae |
Nest in narrow tunnels in dead standing trees, or excavate nests in pith of stems and twigs. Some construct domed nests of mud, plant resins, saps, or gums on the surface of rocks or trees |
| Beetles |
Pollen and nectar as adults; vegetation as larvae or prey such as aphids, slugs, insect eggs |
Larvae overwinter in loose soil or leaf litter Adults shelter under rocks, logs, brush |
- Increase density and diversity of native flowering plants
- Provide refuge from burning and grazing during dormant season and early spring
|
| Butterflies and Moths |
Caterpillar |
Leaves of larval host plants |
Host plants |
- Increase density and diversity of native flowering plants
- Include host plants
- Provide refuge from burning and grazing during dormant season and early spring
|
| Adult |
Nectar; some males obtain nutrients, minerals, and salt from rotting fruit, tree sap, animal dung and urine, carrion, clay deposits, and mud puddles |
Protected site such as a tree, bush, tall grass, or a pile of leaves, sticks, or rocks |
| Flies |
Nectar and sometimes pollen as adults; insect prey such as aphids, scales, mites, thrips |
Larvae found on plants near prey
Pupae and adults overwinter in soil or leaf litter |
- Increase density and diversity of native flowering plants
- Provide refuge from burning and grazing during dormant season and early spring
|
| Hummingbirds |
Nectar, insects, tree sap, spiders, caterpillars, aphids, insect eggs, and willow catkins |
Trees, shrubs, and vines near suitable foraging habitat |
- Increase density and diversity of native flowering plants, particularly species with deep throats
|
| Wasps |
Nectar as adults; insect prey such as caterpillars, aphids, grasshoppers, planthoppers, and true bugs as larvae |
Many nest in the ground; others nest in tunnel nests in wood or cavities in mud or resin |
- Increase density and diversity of native flowering plants
- Provide areas with partially vegetated well-drained soil
- Provide living and dead pithy and woody vegetation
|
[Adapted in part from Marks 2006; Mader et al. 2014]
4.2 Pollinator Habitat Restoration Planning and Design
4.2.1 Site Inventory
Restoration of roadside habitats should begin with an inventory of the baseline site conditions to evaluate what types of restoration actions would be feasible and appropriate. Factors to note may include:
- Baseline Plant Community: When assessing pollen and nectar resources, it is important to look at all of the potential plant resources and which plants are heavily visited by bees and other pollinators. Specifically, it may be useful to note before restoration attempts are made whether there is any remnant plant community at the project site and whether that plant community consists of native, nonnative, or a mixture of native and nonnative plants. At least one study indicates that, with appropriate management, it may be easier in some cases to focus restoration efforts on plant communities that include a mix of native and invasive species rather than attempting to restore a monoculture of invasive plants to a native condition (Davis and Sheley 2011). When evaluating existing plant communities, a special effort should be made to identify very early- and very late-blooming plants. Early flowering plants provide an important food source for bees when emerging from hibernation, and late flowering plants help bumble bees build up their energy reserves before entering winter dormancy (Pywell et al. 2005).
- Slope and Aspect: Roadside vegetation communities provide four distinct microhabitats, consisting of the shoulder, side-slope, ditch, and back-slope areas. These microhabitats have distinct differences in soil conditions, such as gradients in soil moisture, bulk density, organic matter, and pH. These microhabitat differences should be kept in mind when selecting plant materials for restoration (Karim and Mallik 2008).
- Soil Characteristics: Selecting native plant species for restoration based on soil type may be a more useful indicator of roadside restoration establishment success than other criteria, such as historic county-level distribution of the respective plant species (Haan et al. 2012). Soil type is an important consideration when selecting a site, with some plants favoring particular soil textures, such as sand, silt, clay, or loam. Drainage, salinity, pH, organic content, bulk density, and compaction are some of the other factors that influence plant establishment. Many of these factors can be determined from local soil surveys and the National Resources Conservation Service (NRCS) Web Soil Survey (https://websoilsurvey.nrcs.usda.gov/app/). Planning should emphasize those plants that will be adapted for the particular soil conditions faced.
Fertility, soil pathogens, the presence of rhizobium bacteria, and previous herbicide use should also be considered during the planning process (Packard and Mutel 1997). Soil fertility will be most critical during early plant establishment, an important consideration for roadsides created after construction. As the habitat matures, few if any inputs should be required, especially if native plants are selected. Some soil microorganisms, such as rhizobium bacteria, are essential for the successful establishment of certain types of plants, legumes for example. If rhizobium bacteria are absent in the soil, specially inoculated seed is often available. Finally, herbicides such as atrazine and trifluralin can inhibit seed germination (Packard and Mutel 1997). Chemicals and beneficial microorganisms, as well as soil fertility, can be tested by State and extension soil laboratories.
- Adjacent Land Use and Condition: Along with soil and other onsite conditions, adjacent land use and condition should be considered. Many roadsides may already have habitat for native pollinators nearby. These areas of seminatural or natural habitat can significantly increase pollinator populations (Kremen et al. 2004; Williams and Kremen 2007). Adjacent land use may also adversely affect the habitat function of roadsides. For example, even if weeds are eliminated from a roadside restoration site prior to planting, the presence of invasive plants in adjacent lands may result in a persistent problem that requires ongoing management (Steinauer 2003). Adjacent cropland may also present a challenge if the restoration site is not protected from herbicide drift.
- Size: While virtually any size of quality habitat can provide some benefits to pollinators, the larger the planting area, the greater the potential benefit to pollinators and pollination services. To support pollination of adjacent agricultural lands, for example, an area considered for habitat restoration should be at least one-half acre in size, with two acres or more providing even greater benefits (Morandin and Winston 2006; Kremen et al. 2004).
4.2.2 Restoration Design
Restoration design, including the selection of plant species and determination of planting densities, is the next step in the restoration process, following an initial inventory of baseline conditions at a project site. Key factors in the design of restoration efforts include:
- Native Plants: Native plants are advantageous because they generally (1) do not require fertilizers, (2) require fewer pesticides for maintenance, (3) require less water than other nonnative plantings, (4) may function to inhibit nonnative weed encroachment (Falk et al. 2013), (5) provide permanent shelter and food for wildlife, (6) are less likely to become invasive than nonnative plants, (7) promote local native biological diversity (Tinsley et al. 2006), and (8) are preferred by native pollinators (Hopwood 2008; Burghardt et al. 2009; Wu et al. 2009; Williams et al. 2011; Morandin and Kremen 2013a). These factors often make native plantings significantly more cost effective in the long-run.
Research demonstrates the benefits to pollinators of having native wildflowers and grasses on roadsides. Bees and butterflies are more abundant and diverse on roadsides with native plants compared with those dominated by nonnative grass and flowers (Ries et al. 2001; Hopwood 2008).
Beyond benefits to pollinators, native plants may also have a competitive advantage over nonnatives under some revegetation conditions. For example, reseeding of decommissioned roads with native plants resulted in longer term vegetative cover compared with reseeding with nonnative plants (Grant et al. 2011). In another study, the use of wholly native plant seed mixes in Texas resulted in faster and denser ground cover establishment along roadsides than a seed mix consisting of a nonnative species (Cynodon dactylon) that had been selected for rapid establishment and soil stabilization (Tinsley et al. 2006). In that study, the researchers summarize that the nonnative species had greater water requirements during the seedling stage and were less adapted to local site conditions.
- Selecting plants for pollinator attractiveness and bloom time: Ideally, flowers should be available to pollinators throughout the entire growing season. It is desirable to include a diversity of plants with different flower colors, sizes, and shapes as well as varying plant heights and growth habits to encourage the greatest number and diversity of pollinators (Frankie et al. 2002; Potts et al. 2003b; Ghazoul 2006).
Bees typically visit flowers that are purple, violet, yellow, white, and blue (Proctor et al. 1996). Butterflies visit a similarly wide range of colors, including red (Procter et al. 1996), whereas flies are primarily attracted to white and yellow flowers (Stubbs and Chandler 1978). Thus, by having several plant species flowering at once, as well as a sequence of plants flowering through spring, summer, and fall, restored habitat can support a wide range of pollinator species that fly at different times of the season (Feber et al. 1996; Tscharntke et al. 1998; Potts et al. 2003b).
In temperate areas, it may be important to include plants that flower early in the season. For example, early-season pollen and nectar sources may lead to greater reproduction of wild bees, encourage bees that are emerging from hibernation to start their nests nearby, or simply increase the success rate of nearby nests (Carvell et al. 2007). It is also important to include plants that flower late in the season to ensure that bumble bees are strong and numerous going into winter hibernation (Hines and Hendrix 2005; Pywell et al. 2005).
Clusters of single-plant species may also be beneficial where they are possible to create. Research suggests that clump plantings that form a solid block of color when in flower are more attractive to pollinators than species that are widely and randomly dispersed in smaller clumps (Frankie et al. 2002).
- Plant diversity and long-term plant resiliency: Diverse plantings that resemble natural native plant communities are also the most likely to resist pest, disease, and weed epidemics and thus confer the most pollinator benefits over time (Tilman et al. 2006; Oakley and Knox 2013). Species found in association with each other in local natural areas are likely to have the same light, moisture, and nutrient needs such that when these species are put into plantings they are more likely to thrive together (Biondini 2007).
The level of plant community diversity can be measured in several ways. One system used in managed woody plant ecosystems is the 10-20-30 Rule. This rule states that a stable managed plant community (i.e., one that is able to resist insect and disease epidemics) should contain no more than 10 percent of a single plant species, no more than 20 percent of a single genera, and no more than 30 percent of a single family (Santamour 1990).
Diverse plant communities provide higher habitat value for bee pollinators. When multiple species of plants (eight or more) with different bloom times are grouped together at a single site, they tend to attract a significantly greater abundance and diversity of bee species (Frankie et al. 2002). Bee diversity continues to rise with increasing flowering plant diversity (Tscharntke et al. 1998; Carvell 2002; Frankie et al. 2002).
A higher diversity of native plants may help them resist encroachment by invasive weeds. In one study of roadside revegetation, increased plant species diversity was correlated with an increased resistance to invasion by nonnative species (Oakley and Knox 2012). Specifically, research plots planted with 12 species were more susceptible to plant invasion than plots with 24 species.
- Butterfly and moth host plants: Egg-laying sites for butterflies and moths typically consist of plants, upon which the adult will lay eggs and the larvae will feed after hatching (Croxton et al. 2005; Feber et al. 1996). Some butterflies may rely on plants of a single species or genus for host plants (the monarch butterfly is an example, with caterpillars feeding only on species of milkweed [Asclepias spp.]). Others may exploit a wide range of plants, such as some swallowtails (Papilio spp.), whose larvae can feed on a range of trees, shrubs, and forbs (Scott 1986). Given this lifecycle pattern, establishing caterpillar host plants is recognized as a way to sustain butterfly populations (Feber et al. 1996). Roadsides with host plants can support habitat generalist butterflies as well as habitat specialists and migrant species such as the monarch butterfly (Ries et al. 2001). Where possible, revegetation seed mixes and planting plans should include butterfly and moth host plants.
- Inclusion of grasses: Restoration planning usually calls for the inclusion of grasses, sedges, or similar plants in revegetation specifications. Grasses and sedges often provide food or nesting resources for pollinators, such as larval host plants for some butterflies, potential nesting sites for colonies of bumble bees, and possible overwintering sites for various beetles (Kearns and Thompson 2001; Collins et al. 2003; Purtauf et al. 2005).
Most of North America's native bee species (about 70 percent)) nest in the soil (Michener 2007). Bunch grasses tend to provide better nesting habitat than sod-forming grass species. Ground-nesting bees need access to soil surfaces between vegetation to excavate and access their nests (Potts et al. 2005). Roadsides with native bunch grasses had more nesting opportunities for ground-nesting bees and, consequently, a greater abundance of ground-nesting bees (Hopwood 2008).
A combination of grasses and wildflowers will resist weed colonization (Vance et al. 2006). However, it may be important to ensure that grasses do not take over pollinator sites to the exclusion of wildflowers. In the Great Plains, Dickson and Busby (2009) found that wildflower establishment increased with a decrease in grass seeding density. Those findings also suggest that wildflower establishment may be increased by selectively seeding wildflowers into areas with a lower density of dominate grass cover. Another study in Kansas found that the highly competitive growth of one native grass species (Andropogon gerardii) suppressed overall plant diversity, and selective removal of that dominate grass species increased wildflower abundance, diversity, and evenness of cover (McCain et al. 2010).
- Plants to support tunnel-nesting bees: Approximately 30 percent of bees in North America nest as solitary individuals in tunnels in wood, such as abandoned beetle tunnels in logs, stumps, and snags, or excavations in the centers of woody plant stems and twigs (Michener 2007). Some wood-nesters also use materials such as mud, leaf pieces, or tree resin to construct brood cells in their nests (O'Toole and Raw 1999). These bees require woody vegetation to be present on or near the site. Where appropriate, planting native wildflowers with pithy stems, such as cupplant (Silphium perfoliatum), ironweeds (Vernonia spp.) and sunflowers (Helianthus spp.), along with shrubs such as wild rose (Rosa spp.), elderberry (Sambucus spp.), sumac (Rhus spp.), or agave (Agave spp.), will provide resources for stem-nesting bees. In the absence of existing (natural) bee nesting habitat, efforts to restore such habitat by creating brush pile-like structures can, at least in the short term, increase some wild bee numbers (Steffan-Dewenter and Schiele 2008).
- Sourcing plant materials: Plant sourcing issues are associated with the origin of the parent plant material and the type, condition, and relative quality of the material. The source of the plant material can have implications for the quality of the restoration for pollinators.
Where available and economical, native plants and seed should be procured from local ecotype providers (Aldrich 2002; Brandt et al. 2011). Local ecotype plant materials that originated in geographic proximity to the project site will generally establish and grow well because they are adapted to the local climatic conditions (Lippit et al. 1994). Plant material of native species that originated from an area where the climate, moisture, soil, and pest pressures differ may be poorly adapted for local conditions. Additionally, the phenology of non-locally sourced seed can differ (Houseal and Smith 2000; Gustafson et al. 2005). Bloom times of non-locally sourced plants have the potential to be out of sync with pollinators, especially specialist pollinators that are reliant on the pollen from a small subset of plants and time their emergence annually with the bloom time of their host plants. There is some evidence that the use of locally sourced plant material can buffer against invasions (Falk et al. 2013). In contrast, the use of cultivars in plantings can diminish nearby remnant habitat by introducing new diseases or contaminating gene pools (Houseal and Smith 2000). Although the use of locally native seed sources is an ideal, where such sources are not available, eco-regional designations (such as a river valley with little topographical or climatic variation) may be an acceptable way of defining acceptable boundaries for sourcing seed (Miller et al. 2011).
Seed availability and quality may be limited by other factors. Depending on the location, State or local regulations may govern the transfer of plant materials beyond a certain distance (sometimes called Seed Transfer Zones). Commercially procured seed is sometimes certified to guarantee a number of quality standards, including proper species, germination rate, and any amount of weed seed or inert material, but such standards are not universal. Some State Departments of Transportation, such as Florida and Minnesota, are working to increase the availability of local seed in cooperation with local agencies and plant nurseries (Houseal and Smith 2000).
In addition to seed, enhancement sites can be planted with plugs or, in the case of woody plants, container-grown, bare-root, or balled and burlapped materials. Herbaceous plants purchased as plugs have the advantage of rapid establishment and earlier flowering, although the cost of using plugs can be prohibitive in large plantings. Transplanted forbs also typically undergo a period of shock, during which time they may need mulching and supplemental water to ensure survival (Packard and Mutel 1997).
Woody plants may also undergo a period of transplant shock and need similar care. In general, container-grown and balled and burlapped woody plants have a higher survival rate and are available in larger sizes. They are also generally more expensive than bare-root or containerized plants. Containerized trees and shrubs are plants that were either hand-dug from the ground in a nursery setting or harvested as bare-root seedlings, then placed in a container. Such plants should be examined for sufficient root mass before purchase to ensure successful establishment (Shigo 1991).
Beyond the survival issues associated with various types of transplants, the relative value of transplants for restoration should be considered. In one study conducted in Indiana, the use of both transplants and seeding in combination resulted in greater wildflower abundance and diversity compared with seeding of wildflowers alone (Middleton et al. 2010).
Back to top
Chapter 5: Vegetation Management for Pollinators
Most roadsides are subject to some form of vegetation management, whether for aesthetic purposes or for maintaining driver safety, stabilizing slopes and reducing erosion, controlling encroaching woody vegetation and invasive species, or improving or restoring habitat. Roadside vegetation management techniques include mowing, using herbicides, and planting native species. Less frequently used techniques include prescribed burns, grazing, and biological control of invasive exotics. Some roadside managers use a combination of these and other management techniques, with the goal of using ecological principles to manage roadsides sustainably, a practice known as Integrated Roadside Vegetation Management (IRVM).
As reviewed in Chapter 3, roads and roadside vegetation management can have negative impacts on pollinators, particularly those species that are habitat specialists, have low dispersal ability, or have longer generation times. Modifying roadside vegetation management practices can increase the value of roadsides as pollinator habitat and raise the level of pollination services, an outcome that would be particularly beneficial in highly modified landscapes such as agricultural areas. Managing roadsides with pollinators in mind can also benefit other insects that contribute to crop pest control as well as songbirds and game birds.
This section reviews specific roadside vegetation management practices and discusses how they can be modified to reduce impacts on pollinators and increase the value of roadside habitat to pollinators. These modifications range from simple adjustments to the frequency, timing, and scale of management practices, to selecting alternative techniques, to more intensive measures, such as habitat restoration. The management alterations reviewed will benefit pollinators in general, but planning for the management of a site requires extra care when endangered species or species of concern are present. , Assessments of populations of pollinator species can help inform roadside management decisions when included in roadside inventories. Local wildlife agencies and experts can help to determine pollinator species of concern that may be present.
5.1 Managing Roadside Vegetation with Mowing
Mowing has long been used to maintain roadside vegetation, dating as far back as the 1930s when horses pulled the mowers (Harrington 1994; Harper-Lore et al. 2013). Mowing is a relatively straightforward method of cutting roadside vegetation to improve driver sight lines and allow vehicles to pull off the road, if needed. Typically, the vegetation directly adjacent to the road is mown regularly to achieve these safety objectives. The remaining roadside vegetation rarely requires such intensive mowing, though it may be mowed on occasion to prevent the encroachment of woody plants, reduce the chance of uncontrolled wildfires, or control invasive weeds.
When roadside mowing occurs during the growing season, it can directly kill pollinators as well as indirectly cause them harm through changes to the vegetation. Mowing during the growing season can cause direct mortality to pollinators that are in the egg or larval stages because they cannot avoid the mower (Di Giulio et al. 2001; Humbert et al. 2010). This can destroy entire bumble bee colonies (Hatfield et al. 2012). Roadside mowing indirectly affects adult pollinators by temporarily removing flowering plants and butterfly host plants (Johst et al. 2006; Noordijk et al. 2009). For these reasons, mowing should generally be avoided during the growing season, especially if the habitat or vegetation is known to support endangered or rare and sensitive pollinator species (e.g. Karner blue butterfly [Lycaeides melissa samuelis], regal fritillary [Speyeria idalia], rusty-patched bumble bee [Bombus affinis]).
Roadside floral diversity and pollinator diversity are tightly linked (Hopwood 2008); therefore, mowing regimes that encourage plant diversity will most likely benefit pollinators. Frequent mowing reduces native plant growth and the ability of forbs to compete with grasses (Williams et al. 2007). An 18-year mowing treatment experiment in Britain found that the most frequently cut roadsides had the lowest plant diversity (Parr and Way 1988). Frequently mowed ROWs generally have the shortest vegetation, the lowest plant diversity, and lowest amount of nectar, which together result in decreased butterfly abundance (Gerell 1997; Saarinen et al. 2005). In Florida, one study found that frequent mowing limited butterfly numbers all season but was particularly harmful in the fall when populations of several migratory species moved into Florida from northern States (J. Daniels pers. comm.). Higher mowing frequency also appears to increase butterfly roadkill because adult butterflies that are forced to disperse to find new habitat after roadsides are mowed may be more likely to collide with vehicles (Skórka et al. 2013).
In general, infrequent mowing improves the species diversity of grassland habitat. Parr and Way (1988) found roadside plant diversity was highest with two cuts per year (with removal of cut vegetation), one in May and one in August. The May cut was early enough to allow forbs to recover and bloom, while the August cut helped reduce the dominance of certain grasses. In other studies, roadsides that were mown twice a year, early and late in the growing season, had the highest plant diversity and provided the most beneficial conditions for flower-visiting insects (Forman et al. 2003; Noordijk et al. 2009).
Other studies indicate that a single cut per growing season is preferable for plants and pollinators. One study found that mowing twice a year, with the first mowing in June, resulted in fewer flowering species than a single mowing in August (Valtonen et al. 2007). In another study, a mowing regime of twice per year, with the first cut in spring and the second cut mid-summer, during the flight period of the two butterflies studied, was always detrimental at both local and regional scales, while mowing once a year, or every second or third year, before or after the flight period, was much less harmful (Johst et al. 2006).
It is unclear if there is a single best time to mow during the growing season to have the least impact on pollinators. Different studies have identified different times of year depending on the location, climate, and plants examined. Collins et al. (1998) showed that, in the Midwest, mowing once a year in July knocked back dominant grasses and promoted wildflower growth. However, mowing at such a time will limit the growth of fall wildflowers, such as asters and sunflowers, which are not only important forage sources for generalist pollinators but also flowers that some specialist bees preferentially visit and are dependent upon. Roadside mowing in mid-summer may lower the diversity and abundance of butterflies and moths. By delaying mowing until late summer or partially mowing verges, the quality of roadside habitat may be improved for butterflies and moths (Valtonen et al. 2006). Feber et al. (1996) and Johst et al. (2006) recommended a spring or autumn cut over a summer cut for butterflies. However, a late spring cut would affect the less-mobile developmental stages of caterpillars.
Overall, mowing once a year in the early spring or late autumn, when pollinators are less active, or mowing every few years may have the least impact on pollinators. In California or other arid regions, it might be most appropriate to mow after the first rain or when the relative humidity is high to avoid fires started by mowers. Alternatively, mowing could be limited to the strip of vegetation immediately adjacent to the roadside, leaving the remainder of the ROW vegetation and the pollinator populations it supports intact. If weed management is the goal, mowing could be limited to patches of weeds and timed to reduce seed production (Brandt et al. 2011). Mowing techniques that benefit birds and small mammals, such as the use of a flushing bar, reduced mower speed, or a high swath height (Forman et al. 2003), may also benefit pollinators to some degree.
Some States implement high-frequency mowing, thereby maintaining roadside vegetation as turf grass. For example, some of Florida's roads are mown up to 10 times a year at a cost of nearly $13 million, suggesting that a reduction in mowing would provide economic advantages as well as environmental benefits (Harrison 2014). Other State transportation agencies already reduce the frequency of mowing; Montana, Washington, and West Virginia all mow once a year. Minnesota and Michigan enacted a reduced mowing law to encourage wildlife on roadsides. These States allow mowing only once a year, in the late summer, to reduce the mortality of nesting grassland birds (Harper-Lore et al. 2013). Oklahoma delays mowing on roadsides with naturally occurring wildflower populations (i.e., mowing occurs after the seeds of the wildflowers have matured). These sites are identified with signage that indicates that the wildflowers are being protected (Montgomery et al. 2010). Reducing mowing and timing it effectively can improve roadside habitat quality for pollinators.
5.2 Managing Roadside Vegetation with Herbicides
Herbicides are used to control invasive plants, as well as small trees and shrubs, and are important tools for managing roadside habitat. Used indiscriminately, herbicides can directly harm pollinators while also reducing the quality of habitat by removing floral resources and host plants. Overuse of herbicides can also weaken stands of vegetation, making them more vulnerable to weed invasions (Brandt et al. 2011), which also indirectly affects pollinators.
To limit destruction of pollinator host plants or forage plants, avoid broadcast spraying or pellet dispersal. Spot treatment of individual invasive plants with a backpack sprayer, weed wiper, or similar appropriate technology can target weeds without weakening nontarget species (Brandt et al. 2011). For example, targeted spraying of power line ROWs, along with mechanical removal of large shrubs, suppressed undesired vegetation yet maintained diverse habitat for bees (Russell et al. 2005) and butterflies (Forrester et al. 2005). Marrs et al. (1989) recommend a buffer zone of 5 to 10 m for ground sprayers to minimize the risk of herbicide drift onto sensitive vegetation.
Some noninvasive native plants that are valuable for pollinators are often mistakenly identified as weeds. An important step in reducing the impact of herbicides on nontarget plants is to provide applicators with training, with the goal of helping them to identify problem weeds as well as important native vegetation (Brandt et al. 2011). Native thistles (Cirsium spp.), which attract and support many pollinators, including several imperiled species of bumble bees and butterflies, are often mistaken for nonnative invasive thistle species (e.g., musk thistle, Canada thistle) and sprayed with herbicides. The State of Nebraska is home to five species of native thistles and five introduced species that are problematic weeds. The Nebraska Weed Control Association, in cooperation with the Nebraska Department of Agriculture, put together an identification guide to help weed managers avoid treating native thistles (Nebraska Weed Control Association 2014). Milkweeds (Asclepias spp.), which are host plants for monarch butterflies, are another group of plants that are targeted as weeds but do not cause problems on roadsides. Plant identification training will help applicators know what types of habitat (such as roadside prairie or wetland remnants) or stands of native plants (such as milkweeds or thistles) to avoid; it will also help them to keep their applications on target (Brandt et al. 2011; Harper-Lore et al. 2013).
The training provided to applicators can help them determine the preferred herbicides and the best timing for weed control in each situation and improve the efficacy of herbicide use (Brandt et al. 2011). New technologies can target the delivery of herbicides, and GPS/GIS systems can help roadside managers track sensitive sites as well as weed issues (Transportation Research Board 2005). Roadside managers can protect pollinators by targeting herbicide applications, timing applications effectively, and working with adjacent landowners to reduce disturbances that cause weeds. They can also manage herbicide drift (Brandt et al. 2011; Harper-Lore et al. 2013).
5.3 Managing Roadsides by Establishing Native Vegetation
Vegetation used in roadside plantings must achieve multiple goals, including safety, function, and aesthetics (Harrington 1994; Harper-Lore et al. 2013). Additionally, the plants must be able to thrive in the highly disturbed, compacted, and nutrient-poor soil that remains following road construction. There are many native plants that can meet these objectives (Cramer 1991; Quales 2003) and, in the process, provide valuable resources for pollinators.
In many States, turfgrass or introduced grasses such as brome have been used to revegetate roadsides (Quales 2003). Though quick growing, an asset for soil stabilization, these grasses need regular maintenance, including mowing, fertilization, and weed and invasive species control. This maintenance can be costly (e.g. Harrison 2014). Traditionally, it has been thought that the general public preferred a manicured mown turf along the roadside, though there is increasing evidence that motorists may prefer to see roadsides with flowering perennial meadows or flowering shrubs with a mown margin (Lucey and Barton 2011; Barton et al. 2009).
Maintaining existing roadside remnant vegetation is a priority for most DOTs. The Oregon DOT designates Special Management Areas to protect threatened and endangered plants on its land, the Texas DOT uses signage to indicate roadsides with rare plant species, and Wisconsin DOT mows around populations of lupine (Transportation Research Board 2004). Additionally, most DOTs have sought to incorporate native grasses and wildflowers into new roadside plantings to achieve management objectives. The use of native plants is mixed among DOTs, however. Some States, such as Washington, mandate the use of native vegetation through policy or State law (Washington State Department of Transportation 2014). A survey from the Transportation Research Board (2005) reported that several States, including Alaska, Arkansas, Connecticut, Indiana, Montana, Nebraska, New Mexico, Ohio, Texas, Utah, Washington, and West Virginia, used native plants in 80 to 100 percent of revegetation efforts. In contrast, Maine and Maryland used native plants less than 20 percent of the time for revegetation projects as of 2005 (Transportation Research Board 2005). In late 2014, FHWA completed an informal survey of its Division offices about current State DOT vegetation management practices. From the 35 States that responded, 28 DOTs have native seed or wildflower programs and 9 have pollinator-specific programs, including 8 State programs targeted specifically at Monarch conservation (W. Ostrum, pers. comm.)
Designing and establishing site-specific native plantings on roadsides can help roadside managers achieve their management goals. Although introduced species with wide ranges of tolerances are competitive and can establish quickly, they are also more likely to move beyond roadside plantings. Crown vetch (Securigera varia), sweet clover (Melilotus spp.), sericea lespedeza (Lespedeza cuneata), reed canary grass, and smooth brome (Bromus inermis) are just a few examples of introduced plants that were once used on roadsides but became weed problems elsewhere (Harper-Lore and Wilson 2000). In contrast, the use of native grasses and flowers on roadsides can be advantageous because they are adapted to local growing conditions and rarely become weed issues; some species are particularly able to tolerate the poor growing conditions found on roadsides (Harper-Lore and Wilson 2000; O'Dell et al. 2007; Brandt et al. 2011). Stands of native vegetation can also provide stable erosion control and weed suppression because of strong root development (Cramer 1991; Blumenthal et al. 2005; O'Dell et al. 2007). The root systems of native plants also help to reduce runoff in the spring and improve infiltration; this helps replenish groundwater (Harrison 2014; Bugg et al. 1997). In northern States, native grasses act as snow fences in the winter, trapping and preventing snow from blowing across roads (Johnson 2000). DOTs have also begun to view native plantings as an opportunity to showcase a region's natural beauty for tourism, provide a sense of place and heritage, and offer educational opportunities (Harper-Lore and Wilson 2000). Native plants can fill these functional roles while also supporting pollinators.
Seeding roadsides with native vegetation can increase the diversity of plants in the local area and, in turn, benefit pollinators and other wildlife (Forman et al. 2003). Native plants support more pollinators than do nonnative plants (French et al. 2005; Tallamy and Shropshire 2009; Williams et al. 2011; Morandin and Kremen 2013a), and research demonstrates the benefits to pollinators of having native wildflowers on roadsides. For example, bees were twice as abundant and 35 percent more diverse on Kansas roadsides with native plants compared with those dominated by nonnative grass and flowers; roadsides with native plants also had more nesting opportunities for ground-nesting bee species (Hopwood 2008). Ries et al. (2001) compared roadsides in Iowa that had been replanted with native prairie grasses and wildflowers with grass- or weed-dominated roadsides and found that habitat-sensitive butterfly species such as the regal fritillary and Delaware skipper (Anatrytone logan) were significantly more common in prairie roadsides.
Public education is a key component of vegetation management through the use of native plants. Plantings may take several years to establish, and educating the public on the economic and ecological value of establishing and maintaining native vegetation on roadsides, including the benefits to pollinators, will increase acceptance of the practice (Harrington 1994).
5.4 Use of Fire on Roadsides
Prescribed burning is used to manage roadside vegetation in regions where the native grassland and savanna communities evolved. Prescribed burns can aid in native plant establishment and control invasive species. Fire may also benefit sites that are being encroached upon by woody plants or sites where grasses have crowded out wildflowers. For example, prescribed burns can revitalize roadside prairie vegetation (Schramm 1990). Prescribed burns are not ecologically appropriate for every region or site; they may be an air quality concern or compromise highway safety at certain sites. States that have used prescribed burns on roadsides include California, Florida, Iowa, Minnesota, and Wisconsin (Transportation Research Board 2005). Other States, such as Kansas, have permitted a trial use of fire on a limited basis.
Fire supports the pollinator community by maintaining the open plant community, which is composed primarily of the flowering species upon which pollinators depend. Pollinator abundance decreases with woody cover (Campbell et al. 2007) and increases with forb density post burn (Van Nuland et al. 2013). Yet prescribed burns can be harmful to many pollinators and have long-term impacts on the populations of some species. See Section 3.1.4 for more information on prescribed burns and their impact on pollinators. Modifying the use of fire as a roadside management tool by timing burn events seasonally or limiting the scale and frequency of the burns will help to make this management practice more pollinator friendly.
The timing of a burn is important with respect to its impact on the roadside plant community. Intense summer fires are most efficient at controlling woody species such as red cedar; dormant-season burns in fall or winter encourage the growth of cool-season grasses. Burns at either of these times affects pollinators. Summer burns remove vegetation at a time when pollinators need floral resources, host plants, and nesting materials, and winter burns destroy species that overwinter in leaf litter or stems. Burning an entire roadside corridor runs the risk of extirpating the local pollinator community. By leaving adequate refuge habitat, enough pollinators remain to recolonize the burned areas, thereby sustaining the temporary decrease in habitat quality. Rotational burning of small sections of roadsides every 3 to 5 years that leaves refuge habitat provides the benefits of prescribed fire without causing irreparable damage to the local pollinator community (Black et al. 2011).
5.5 Use of Grazing on Roadsides
Historically, grazing was regularly used to manage roadsides, particularly in the eastern United States (Transportation Research Board 2005). As vehicle speeds increased, routine grazing was phased out. Currently, grazing is more often used as a component of an IRVM program rather than as a stand-alone strategy. The advantages of using grazing on roadsides include the ability to control large infestations of weeds as well as weed control in inaccessible spots such as steep slopes or near water. Goats and sheep, which can be herded, are most often used for roadside grazing. Goats and sheep prefer to eat broadleaf plants and are therefore used in some settings for conservation goals, such as the removal of invasive forbs (DiTomaso 2000). Goats and sheep have been used on roadsides to control weeds such as leafy spurge, knapweed, kudzu, and Himalayan blackberry. New Mexico DOT has permitted a trial use of goat grazing on roadsides to control noxious weeds (Transportation Research Board 2005). Goats were also used to remove invasive shrubs on a Maryland roadside that was populated by an endangered species of turtle (Harper-Lore et al. 2013).
Livestock grazing can adversely affect pollinator populations directly by trampling (Warren 1993) and indirectly by altering plant community composition (Stoner and Joern 2004). Livestock grazing can remove pollen and nectar resources, as well as host plants (Hayes and Holl 2003), and destroy bee nests (Sugden 1985) (see Section 3.1.3 for more information on grazing threats). However, pollinators can show a positive response to light or moderate grazing when it is used to maintain favorable vegetation heights as well as habitat heterogeneity, which is favorable to some butterflies (Ravenscroft 1994; Thomas and Jones 1993; Davies et al. 2005). For grazing to have minimal impacts on pollinators, there must be careful consideration of specialist or rare pollinators as well as the timing, intensity, and duration of grazing (Black et al. 2011).
Grazing plans should be site specific and carefully timed to encourage the grazers to feed selectively on the undesirable species. For example, grazing should occur when the weed is palatable. However, the introduction of grazers should be timed carefully, balancing the need to control weeds with the needs of pollinators. If habitat specialist pollinator species are present, grazing should not take place during the adult flight period. Grazing during periods when floral resources are already scarce (e.g., mid-summer) may result in insufficient forage for pollinators such as bumble bees, which need forage in late summer and fall (Carvell 2002).
The duration of grazing will depend on the density of grazers. If stocking density is high, the grazers will need to be on the roadside for short periods of time. Hatfield and LeBuhn (2007) found that uncontrolled sheep grazing removed enough flowering plants to eliminate bumble bees from some study sites. Generally speaking, grazing periods should be short, with relatively long recovery periods for the habitat. Grazing is not right for every roadside locale, but when the grazing plan suits local conditions, it can be compatible with pollinator support.
5.6 Use of Biological Control on Roadside Weeds
Biological control of weeds involves releasing natural enemies of a target weed species. Natural enemies of invasive weeds, such as insects or pathogens, are typically introduced from the region of the weed's origin. Independent experts, along with the U.S. Department of Agriculture, first screen natural enemies to determine their suitability for introductions. Once this extensive screening process is complete, the U.S. Department of Agriculture's Animal and Health Inspection Service, along with a State's Department of Agriculture or Department of Natural Resources, is responsible for importing, multiplying, and releasing the natural enemies. The use of biological control on roadsides can inhibit the growth of targeted weeds, reduce herbicide use (Harper-Lore et al. 2013), and, consequently, reduce the impacts of herbicides on pollinators. Biological control is particularly convenient on inaccessible roadside sites with large weed infestations. Additionally, biological control is a relatively inexpensive investment that can provide widespread, long-term control. Because biological control is a more targeted approach to weed control, roadside pollinator habitat would most likely not be altered to the same extent as it would under other forms of vegetation management. However, one disadvantage of this type of biological control is the potential for unpredictable and irreversible ecological consequences, such as introducing a species that is outside of its natural range. For example, the Eurasian weevil (Rhinocyllus conicus) was introduced to control musk thistle (Carduus nutans); however, it switched hosts and began to feed on native thistles, including rare species (Louda et al. 1997). The loss of native thistles or other native species can indirectly harm pollinators that rely on these plants for pollen and nectar.
Biological control is the least widely implemented roadside management technique; however, some DOTs, including Florida, Illinois, Kentucky, Maryland, Utah, and Washington, have implemented biological control (Transportation Research Board 2005). For example, Minnesota's DOT has released beetles to control purple loosestrife (Lythrum salicaria) and leafy spurge (Euphorbia esula) (Johnson 2000). New Hampshire's DOT has released Gallerucella beetles to control purple loosestrife (Harper-Lore et al. 2013), and California's DOT has research trials under way to test biological control agents for yellow star thistle (Centaurea solstitialis) and Russian thistle (Salsola kali) (Smith 2006).
5.7 Incorporating All the Tools: Integrated Roadside Vegetation Management
IRVM is an outgrowth of Integrated Pest Management (IPM), a science-based framework for making decisions about pest control. IPM programs involve identifying pest problems, establishing thresholds at which treatment is needed, and then utilizing a variety of biological, cultural, mechanical, and chemical tools in the most sustainable manner possible. In the same way that IPM is an alternative to routine calendar-based insecticide spraying on crops, IRVM is an alternative to intensive mowing and blanket herbicide spraying on roadsides. IRVM incorporates the planting of native vegetation with site-appropriate management strategies to achieve cost-effective and more environmentally sustainable management practices for roadsides. The National Roadside Vegetation Management Association defines IRVM as “a decision-making and quality management process for maintaining roadside vegetation that integrates the needs of local communities and highway users; knowledge of plant ecology (and natural processes); design, construction, and maintenance considerations; monitoring and evaluation procedures; government statutes and regulations, and technology with cultural, biological, mechanical, and chemical pest control methods to economically manage roadsides for safety plus environmental and visual quality” (Harper-Lore et al. 2013).
Because IRVM involves tailoring management to a specific site, an IRVM program can be very compatible with pollinator protection. By encouraging plant diversity and reducing mowing and herbicide use, IRVM improves the quality of the roadside habitat. IRVM practices that limit disturbance but maintain plant diversity, such as spot mowing to reduce weed seed production, limited grazing, occasional prescribed fire, and biological control, most likely also benefit pollinators. Species mixes that have been designed for IRVM plantings can fulfill functional roles that are valuable to roadside vegetation management while supporting pollinators. For example, Iowa's IRVM manual (Brandt et al. 2011) recommends selecting native grasses and wildflowers that establish quickly to provide soil stabilization, cool- and warm-season grasses that buffer invasive species, and wildflowers that bloom throughout the growing season for wildlife as well as aesthetics. Roadsides with wildflowers that have sequential and overlapping bloom times would be able to support pollinators that emerge at various times in the growing season (e.g., regal fritillary butterflies) as well as pollinators with flight seasons that extend from spring to fall (e.g., bumble bees). The only study that explicitly investigated the effects of IRVM on butterflies found that IRVM practices in Iowa resulted in roadsides with more host plants and more nectar plants for butterflies (Ries et al. 2001).
Some States implement IRVM programs by official policy (e.g., Florida, Iowa, Maryland, Minnesota, New York, Pennsylvania, Texas), while others implement it without a policy requirement (e.g., Nebraska, South Carolina, Utah, and West Virginia) (Transportation Research Board 2005). Florida DOT estimates an annual cost of at least $33.3 million to maintain ROWs (Harrison 2014). However, through the implementation of sustainable management practices, Florida DOT estimates that vegetation management costs can be reduced by at least 30 percent (Harrison 2014). Florida DOT also identified roadside functions that can produce additional savings; these are related to pollination services, reduced stormwater flow, carbon sequestration, air quality, invasive species resistance, and aesthetics. IRVM training and resources in States that currently do not implement IRVM may help reduce roadside maintenance costs while supporting pollinators.
Back to top
Chapter 6: Case Studies
6.1 Bringing Prairie Back to Iowa: Iowa's Integrated Roadside Vegetation Management Program and Living Roadway Trust Fund
Prairie once dominated Iowa's landscape, covering more than 85 percent of the State. With less than 0.1 percent of virgin prairie remaining, and more than 95 percent of Iowa's original wetlands destroyed, Iowa is the nation's most altered landscape. Prior to the mid-1980s, roadside weed control in Iowa relied heavily on blanket spraying, putting large amounts of herbicide into the environment with undesirable consequences. Recognizing Iowa's lost heritage and the need to protect groundwater and surface waters, the Iowa roadside managers began making some changes. For example, it began using native prairie grasses and wildflowers for erosion control and reintroduced “a little wildness,” according to Kirk Henderson, retired from the Native Roadside Vegetation Center at the University of Northern Iowa.
In 1989, the Iowa legislature passed integrated roadside vegetation management (IRVM) legislation that created roadsides program to promote an ecologically integrated approach to roadside management while maintaining a safe travel environment (Code of Iowa, Section 314). The legislation emphasized the establishment and protection of native vegetation as well as judicious use of herbicides, mowing, prescribed burning, and other management tools. Iowa is widely seen as a leader in IRVM, in large part because of this legislation. The bill also established the Living Roadway Trust Fund, an annual competitive grant program administered by the Iowa DOT that provides funding for school, city, county and State projects, as well as research projects involving IRVM. Iowa's road use tax, along with several other sources, funds the Living Roadway Trust Fund. Roadside managers can submit applications to obtain resources to help them implement IRVM, including vegetation inventories, native seed, equipment for burns or plant establishment, GPS units, signage, workshops, and more. Roadsides are seeded with mixes of species that are appropriate for a particular site, including many wildflowers that are attractive to pollinators. Seed mixes also contain species that bloom at different times throughout the growing season, which helps support pollinators all season long. The targeted vegetation management practiced by Iowa's roadside managers also benefits pollinators (Ries et al. 2001).
Research projects have also been supported by the Living Roadway Trust Fund, including studies of restoration techniques as well as studies of the impact of roadside habitat on butterflies (Ries et al. 2001) and bees (Hopwood et al. 2010). Since the bill, more than 100,000 acres of Iowa's nearly 600,000 acres of State and county roadsides have been planted to native vegetation (Brandt et al. 2011). In the process, Iowa has created experienced roadside managers who are equipped to collaborate with other land managers around the State and bring habitat, and wildlife such as pollinators, back to Iowa's landscape.
6.2 Monarch-Friendly Roadside Management: Roadsides for Wildlife Program, Minnesota
Transportation corridors are a significant, yet often overlooked, opportunity for monarch conservation. Monarchs are typically present in Minnesota from May through early September, and a drive across most rural Minnesota landscapes immediately reveals roadside milkweeds as a prime resource in supporting that population.
Recognizing roadsides as important resources, the Minnesota Department of Natural Resources (DNR) Roadsides for Wildlife Program has engaged rural landowners and State and local transportation agencies and presented them with a comprehensive set of management recommendations, which are intended to protect plants and wildlife while also balancing the need for road safety. Among these recommendations are:
- Using native prairie plants for roadside revegetation. In Minnesota, more than 500,000 acres of roadsides are available for wildlife habitat in just the southern two-thirds of the State, a region that includes prime areas of pheasant habitat. The DNR encourages transportation agencies to replant these areas with native grasses and wildflowers whenever they are disturbed for routine maintenance or new road construction. In addition to supporting monarchs and other wildlife, these deep-rooted native plants increase infiltration, capture runoff from nearby farmlands, and improve aesthetics. Working with the Minnesota State Department of Transportation and county and township highway departments, the DNR has completed hundreds of roadside prairie habitat restoration projects across the State, totaling thousands of acres of restored habitat. Many of these efforts include milkweeds.
- Delaying mowing of roadside ditch bottoms and back slopes until after August 1. Although intended to protect ground-nesting birds, late-season mowing also provides a longer period of time for monarch caterpillars to develop and extends the availability of nectar plants later into the summer. From a monarch conservation perspective, mowing would ideally be delayed further into the fall, until migrating monarchs have left the State, but the recommendations recognize the need some transportation managers have to maximize mowing opportunities with limited staff resources. Widespread herbicide spraying and burning are not recommended in ditch areas.
- Using shoulder and spot mowing to manage invasive species, control safety concerns, and prevent snow drifting. The DNR points out to program participants that widespread mowing and herbicide spraying are not only detrimental to wildlife but are also expensive and often unnecessary. By focusing on problem spots, transportation agencies can save money and time.
For more information about Minnesota's Roadsides for Wildlife Program, visit http://www.dot.state.mn.us/roadsides/vegetation/.
Back to top
Chapter 7: Literature Cited
7.1 Printed References
Aldrich, J. H. 2002. Factors and benefits in the establishment of modest-sized wildflower plantings: A review. Native Plants Journal 3(1):67-86.
Allen-Wardell, G., P. Bernhardt, R. Bitner, A. Burquez, S. Buchmann, J. Cane, P. Cox, V. Dalton, P. Feinsinger, M. Ingram, D. Inouye, C. E. Jones, K. Kennedy, P. Kevan, H. Koopowitz, R. Medellin, S. Medellin-Morales, G. Nabhan, B. Pavlik, V. Tepedino, P. Torchio, and S. Walker. 1998. The potential consequences of pollinator declines on the conservation of biodiversity and stability of food crop yields. Conservation Biology 12(1):8-17.
Alston, D. G., and V. J. Tepedino. 2000. Direct and indirect effects of insecticides on native bees. In G. L. Cuningham and M. W. Sampson (Technical Coordinators), Grasshopper Integrated Pest Management User Handbook. United States Department of Agriculture Animal and Plant Health Inspection Services Technical Bulletin No. 1809. Washington, DC.
Altizer S. M. and K. S. Oberhauser. 1999. Effects of the protozoan parasite Ophryocystis elektroscirrha on the fitness of monarch butterflies (Danaus plexippus). Journal of Invertebrate Pathology 74:76-88.
Altizer S. M., K. S. Oberhauser, and L. P. Brower. 2000. Associations between host migration and the prevalence of a protozoan parasite in natural populations of adult monarch butterflies. Ecological Entomology 25:125-139.
Araratyan, L. A. and S. A. Zakbaryan. 1988. On the contamination of snow along main highways. Biologicheskii Zhurnal Armenii 41: 511-519.
Arita, H. T., and D. E. Wilson. 1987. Long-nosed bats and agaves: the tequila connection. Bats 5(4), 3-5.
Arizona Game and Fish Department. 2003. Lesser Long-nosed Bat. Unpublished abstract compiled and edited by the Heritage Data Management System, Arizona Game and Fish Department, Phoenix, AZ. 1-9 pp.
Balmer, O. and A. Erhardt. 2000. Consequences of succession on extensively grazed grasslands for central European butterfly communities: rethinking conservation practices. Conservation Biology 14:746-757.
Baron, G. L., N. E. Raine, and M. J. Brown. 2014. Impact of chronic exposure to a pyrethroid pesticide on bumble bees and interactions with a trypanosome parasite. Journal of Applied Ecology 51(2): 460-469.
Bartomeus, I., J. S. Ascher, J. Gibbs, B. N. Danforth, D. L. Wagner, S. M. Hedtke, and R. Winfree. 2013. Historical changes in northeastern US bee pollinators related to shared ecological traits. Proceedings of the National Academy of Sciences 110:4656-4660.
Barton, S., R. Darke, and G. Schwetz. 2009. Enhancing Delaware Highways. Delaware Department of Transportation. Roadside Vegetation Establishment and Management Manual. Available: https://deldot.gov/Publications/manuals/edh/index.shtml?dc=edhEM. Accessed November 2014.
Bawa, K. S. 1990. Plant-pollinator interactions in tropical rain forests. Annual Review of Ecology and Systematics: 399-422.
Bee Informed Partnership. 2014. Bee Informed Partnership ¦ Using beekeepers' real world experience to solve beekeepers' real world problems. Available: https://beeinformed.org/. Accessed April 23, 2014.
Belsky, A., A. Matzke, and S. Uselman. 1999. Survey of livestock influences on stream and riparian ecosystems in the western United States. Journal of Soil and water Conservation 54:419-431.
Bhattacharya, M., R.B. Primack, and J. Gerwein. 2003. Are roads and railroads barriers to bumble bee movement in a temperate suburban conservation area? Biological Conservation 109: 37-45.
Biesmeijer J. C., S. P. M. Roberts, M. Reemer, R. Ohlemüller, M. Edwards, T. Peeters, A. P. Schaffers, S. G. Potts, R. Kleukers, C. D. Thomas, J. Settele, and W. E. Kunin. 2006. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313:351- 354.
Biondini, M. 2007. Plant Diversity, Production, Stability, and Susceptibility to Invasion in Restored Northern Tall Grass Prairies (United States). Restoration Ecology 15:77-87.
Blaauw, B. R. and R. Isaacs. 2014. Flower plantings increase wild bee abundance and the pollination services provided to a pollination-dependent crop. Journal of Applied Ecology.
Black, S. H., M. Shepherd, and M. Vaughan. 2011. Rangeland management for pollinators. Rangelands 33(3):9-13.
Black, S., C. Fallon, C., Hatfield, R. and C. Mazzacano. 2014. Controlled Burning and Mardon Skipper: Summary of Mardon Skipper Coon Mountain Burn Site Occupancy Study Data from 2009 to 2013. Report to the U.S. Forest Service, Oregon Zoo, and U.S. Fish and Wildlife Service: 29.
Blumenthal, D. M., N. R. Jordan, and E. L. Svenson. 2005. Effects of prairie restoration on weed invasions. Agriculture, Ecosystems and Environment 107:221-230.
Bogemams, J., L. Nicrinck, and J. M. Stassart. 1989. Effect of deicing chloride salts on ion accumulation in spruce (Picea abies (L.) sp.). Plant and Soil 113:3-11.
Boggs, C. L., and K. D. Freeman. 2005. Larval food limitation in butterflies: effects on adult resource allocation and fitness. Oecologia 144(3): 353-361.
Bonmatin, J. M., I. Moineau, R. Charvet, C. Fleche, M. E. Colin, and E. R. Bengsch. 2003. A LC/APCI-MS/MS method for analysis of imidacloprid in soils, in plants, and in pollens. Analytical Chemistry 75:2027-2033.
Bonmatin, J. M., I. Moineau, R. Charvet, M. E. Colin, C. Fleche, and E. R. Bengsch. 2005. Behaviour of imidacloprid in fields. Toxicity for honey bees. In E. Lichtfouse, J. Schwarzbauer, and D. Robert (ed.), Environmental Chemistry, Green Chemistry and Pollutants in Ecosystems, , 483-494. New York: Springer.
Bosch, J. and W. P. Kemp. 2001. How to manage the blue orchard bee as an orchard pollinator. Available: . Accessed March 31, 2014.
Brandt, J., K. Henderson, and J. Uthe. 2011. Integrated Roadside Vegetation Management Technical Manual. Available: https://www.tallgrassprairiecenter.org/irvm. Accessed November 2014.
Brosi, B. J., and H. M. Briggs. 2013. Single pollinator species losses reduce floral fidelity and plant reproductive function. Proceedings of the National Academy of Sciences 110(32):13044-13048.
Brosi, B. J., G. C. Daily, T. M. Shih, F. Oviedo, and G. Durán. 2008. The effects of forest fragmentation on bee communities in tropical countryside. Journal of Applied Ecology 45(3):773-783.
Brower, L. P., O. R. Taylor, E. H. Williams, D. A. Slayback, R. R. Zubieta, and M. I. Ramírez. 2012a. Decline of monarch butterflies overwintering in Mexico: is the migratory phenomenon at risk? Insect Conservation and Diversity 5:95-100.
Brower, L. P., O. R. Taylor, and E. H. Williams. 2012b. Response to Davis: Choosing relevant evidence to assess monarch population trends. Insect Conservation and Diversity 5:327-329.
Brown, J. J. 1987. Toxicity of herbicides thiobencarb and endothall when fed to laboratory-reared Trichoplusia ni (Lepidoptera: Noctuidae). Pesticide Biochemistry and Physiology 27:97-100.
Brunzel, S., H. Elligsen, and R. Frankl 2004. Distribution of the Cinnabar moth Tyria jacobaeae L. at landscape scale: use of linear landscape structures in egg laying on larval hostplant exposures. Landscape Ecology 19(1), 21-27.
Buchmann, S. L. 1983. Buzz pollination in angiosperms, p. 73-113. In Handbook of Experimental Pollination Biology, C. E. Jones and R. J. Little, (eds.), Van Nostrand Reinhold, New York
Bugg, R. L., C. S. Brown, and J. H. Anderson. 1997. Restoring native perennial grasses to rural roadsides in the Sacramento Valley of California: Establishment and evaluation. Restoration Ecology 5:214-228.
Burghardt, K. T., D. W. Tallamy, and W. G. Shriver. 2009. Impact of native plants on bird and butterfly biodiversity in suburban landscapes. Conservation Biology 23:219-224.
Calder, W. A. 2004. Rufous and broad-tailed hummingbirds—Pollination, migration, and population biology. Pp. 59-79 in Conserving Migratory Pollinators and Nectar Corridors in Western North America, G. P. Nabhan, ed. Tucson: University of Arizona Press.
Calderone, N. W. 2012. Insect Pollinated Crops, Insect Pollinators and US Agriculture: Trend Analysis of Aggregate Data for the Period 1992-2009. PLOS ONE 7:e37235. DOI: 10.1371/journal.pone.0037235
Cameron, S. A., J. D. Lozier, J. P. Strange, J. B. Koch, N. Cordes, L. F. Solter, and T. L. Griswold. 2011. Patterns of widespread decline in North American bumble bees. Proceedings of the National Academy of Sciences 108(2): 662-667.
Campbell, J. W., J. L. Hanula, and T. A. Waldrop. 2007. Effects of prescribed fire and fire surrogates on floral visiting insects of the blue ridge province in North Carolina. Biological Conservation 134(3):393-404.
Cane, J. H. 2001. Habitat fragmentation and native bees: a premature verdict? Conservation Ecology 5(1):3. Available: https://www.ecologyandsociety.org/vol5/iss1/art3/.
Cane, J. H., and D. Schiffhauer. 2003. Dose-response relationships between pollination and fruiting refine pollinator comparisons for cranberry (Vaccinium macrocarpon). American Journal of Botany 90:1425-1432.
Cane, J. H., and J. L. Neff. 2011. Predicted fates of ground-nesting bees in soil heated by wildfire: Thermal tolerances of life stages and a survey of nesting depths. Biological Conservation 144(11):2631-2636.
Caron, D. M., R. C. Lederhouse, and R. A. Morse. 1975. Insect pollinators of onion in New York State. HortScience 10:273-274.
Carvell, C. 2002. Habitat use and conservation of bumble bees (Bombus spp.) under different grassland management regimes. Biological Conservation 103:33-49.
Carvell, C. W., R. Meek, R. F. Pywell, D. Goulson, and M. Nowakowski. 2007. Comparing the efficacy of agri-environment schemes to enhance bumble bee abundance and diversity on arable field margins. Journal of Applied Ecology 44:29-40.
Christen, D. C., and G. R. Matlack. 2009. The habitat and conduit functions of roads in the spread of three invasive plant species. Biological Invasions 11: 453-465.
Coffin, A. W. 2007. From roadkill to road ecology: A review of the ecological effects of roads. Journal of Transport Geography 15:396-406.
Colla, S. R., and C. M. Ratti. 2010. Evidence for the decline of the western bumble bee (Bombus occidentalis Greene) in British Columbia. Pan-Pacific Entomologist 86:32.
Colla, S. R., and L. Packer. 2008. Evidence for decline in eastern North American bumble bees (Hymenoptera: Apidae), with special focus on Bombus affinis Cresson. Biodiversity and Conservation 17:1379-1391.
Colla, S. R., M. C. Otterstatter, R. J. Gegear, and J. D. Thomson. 2006. Plight of the bumble bee: pathogen spillover from commercial to wild populations. Biological Conservation 129:461-467.
Colla, S., F. Gadallah, L. Richardson, D. Wagner, and L. Gall. 2012. Assessing declines of North American bumble bees (Bombus spp.) using museum specimens. Biodiversity and Conservation 21(14):3585-3595.
Collins, K. L., N. D. Boatman, A. Wilcox, and J. M. Holland. 2003. Effects of different grass treatments used to create overwintering habitat for predatory arthropods on arable farmland. Agriculture, Ecosystems & Environment 96:59-68.
Collins, S. L., A. K. Knapp, J. M. Briggs, J. M. Blair, and E. M. Steinauer. 1998. Modulation of diversity by grazing and mowing in native tallgrass prairie. Science 280:745-747.
Corbet, S. A., M. Fussell, R. Ake, A. Fraser, C. Gunson, A. Savage, and K. Smith. 1993. Temperature and the pollinating activity of social bees. Ecological Entomology 18:17-30.
Cramer, C. 1991. Tougher than weeds: native prairie plants, better management trim roadside spraying 90%. The New Farm 13:37-39.
Croxton, P. J., J. P. Hann, J. N. Greatorex-Davis, and T. H. Sparks. 2005. Linear hotspots? The floral and butterfly diversity of green lanes. Biological Conservation 121:579-584.
Dale, J. M. and B. Freedman. 1982. Lead and zinc contamination of roadside soil and vegetation in Halifax, Nova Scotia. Proceedings of the Nova Scotian Institute of Science 32:327-336.
Dana, R. 1997. Characterization of three Dakota skipper sites in Minnesota. Unpublished report, Minnesota Department of Natural Resources, Natural Heritage and Nongame Research Program, St. Paul, MN. Available:
http://files.dnr.state.mn.us/eco/nongame/projects/consgrant_reports/1997/1997_dana.pdf. Accessed January 2, 2013.
Davies, Z. G., R. J. Wilson, T. M. Brereton, and C. D. Thomas. 2005. The re-expansion and improving status of the silver-spotted skipper butterfly (Hesperia comma) in Britain: a metapopulation success story. Biological Conservation 124:189-198.
Davis, K. and R. Sheley. 2011. Promoting Native Vegetation and Diversity in Exotic Annual Grass Infestations. Restoration Ecology 19:159-165.
Decourtye, A., C. Armengaud, M. Renou, J. Devillers, S. Cluzeau, M. Gauthier, and M. Pham-Delegue. 2004. Imidacloprid impairs memory and brain metabolism in the honeybee (Apis mellifera L.). Pesticide Biochemistry and Physiology 78:83-92.
Decourtye, A., J. Devillers, E. Genecque, K. Le Menach, H. Budzinski, S. Cluzeau, and M.H. Pham-Delegue. 2005. Comparative sublethal toxicity of nine pesticides on olfactory performances of the honeybee Apis mellifera. Pesticide Biochemistry and Physiology 78:83-92.
Desneaux, N., A. Decourtye, and J. Delpuech. 2007. The sublethal effects of pesticides on beneficial arthropods. Annual Review of Entomology 52:81-106.
Di Giulio, M., P. J. Edwards, and E. Meister. 2001. Enhancing insect diversity in agricultural grasslands: the roles of management and landscape structure. Journal of Applied Ecology 38(2):310-319.
Dickson, T. L. and W. H. Busby. 2009. Forb species establishment increases with decreased grass seeding density and increased forb seeding density in a northeast Kansas, U.S.A., experimental prairie restoration. Restoration Ecology 17:597-605.
Dirig, R. and J. F. Cryan 1991. The status of silvery blue subspecies (Glaucopsyche lygdamus lygdamus and G. l. couperi: Lycaenidae) in New York. Journal of the Lepidopterists' Society 45(4):272-290..
DiTomaso, J. M. 2000. Invasive weeds in rangelands: species, impacts, and management. Weed Science 48:255-265.
Dover, J., N. Sotherton, and K. Gobbett. 1990. Reduced pesticide inputs on cereal field margins: the effects on butterfly abundance. Ecological Entomology 15:17-24.
Dramstad, W. E. 1996. Do bumble bees (Hymenoptera: Apidae) really forage close to their nests? Journal of Insect Behavior 9(2): 163-182.
Evans, E., R. Thorp, S. Jepsen, and S. Hoffman Black. 2008. Status review of three formerly common species of bumble bee in the subgenus /Bombus/. The Xerces Society. 63 pp. Available: http://www.xerces.org/wp-content/uploads/2009/03/xerces_2008_bombus_status_review.pdf.
Falk, A. D., T. E. Fulbright, F. S. Smith, L. A. Brennan, A. J. Ortega-Santos, and S. Benn. 2013. Does Seeding a Locally Adapted Native Mixture Inhibit Ingress by Exotic Plants? Restoration Ecology 21(4):474-480.
Feber, R. E., H. Smith, and D. W. Macdonald. 1996. The effects on butterfly abundance of the management of uncropped edges of arable fields. Journal of Applied Ecology 33:1191-1205.
Fiedler, A. K., D. A. Landis, and M. Arduser. 2012. Rapid shift in pollinator communities following invasive species removal. Restoration Ecology 20(5):593-602.
Fleming, T. H., C. Geiselman. and W. J. Kress. 2009. The evolution of bat pollination: a phylogenetic perspective. Annals of Botany 104: 1017-1043.
Flockhart, T. D. T., J. B. Pichancourt, D. Ryan Norris, and T. G. Martin. 2014. Unraveling the annual cycle in a migratory animal: Breeding-season habitat loss drives population declines of monarch butterflies. Journal of Animal Ecology DOI: 10.1111/1365-2656.12253.
Fontaine, C., I. Dajoz, J. Meriguet, and M. Loreau. 2005. Functional diversity of plant-pollinator interaction webs enhances the persistence of plant communities. PLOS Biology 4(1), e1. DOI: 10.1371&347;journal.pbio.0040001
Forman, R. T. T., D. Sperling, J. A. Bissonette, A. P. Clevenger, C. D. Cutshall, V. H. Dale, L. Fahrig, R. France, C. R. Goldman, K. Heanue, J. A. Jones, F. J. Swanson, T. Turrentine, and T. C. Winter. 2003. Road Ecology: Science and Solutions. Washington, D.C.: Island Press.
Forrester, J. A., D. J. Leopold, and S. D. Hafner. 2005. Maintaining critical habitat in a heavily managed landscape: effects of power line corridor management on Karner blue butterfly (Lycaeides melissa samuelis) habitat. Restoration Ecology 13(3):488-498.
Frankie, G. W., R. W. Thorp, M. H. Schindler, B. Ertter, and M. Przybylski. 2002. Bees in Berkeley? Fremontia 30(3-4):50-58.
Frankie, G. W., S. B. Vinson, L. E. Newstrom, J. F. Barthell, W. A. Haber, and J. K. Frankie. 1990. Plant phenology, pollination ecology, pollinator behaviour and conservation of pollinators in Neotropical dry forest. In K. S. Bawa and M. Hadley (Ed.), Reproductive ecology of tropical forest plants, , 37-47. The Parthenon Publishing Group, Paris.
Free, J. B., D. Gennard, J. H. Stevenson, and I. H. Williams. 1975. Beneficial insects present on a motorway verge. Biological Conservation 8(1):61-72.
French, K., R. Major, and K. Hely. 2005. Use of native and exotic garden plants by suburban nectarivorous birds. Biological Conservation 121:545-559.
Fried, J. H., D. J. Levey, and J. A. Hogsette. 2005. Habitat corridors function as both drift fences and movement conduits for dispersing flies. Oecologia 143(4):645-651.
Fry G. L. A. and W. G. Robson. 1994. Effects of field margins on butterfly movement. In: Boatman ND (ed), Field margins: integrating agriculture and conservation. British Crop Protection Council monograph, no. 58. BCPC, Thornton Heath, Surrey, pp 111-116.
Garibaldi, L. A., I. Steffan-Dewenter, R. Winfree, M. A. Aizen, R. Bommarco, S. A. Cunningham, C. Kremen. 2013. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 339(6127):1608-1611.
Gelbard, J. L. and J. Belnap. 2003. Roads as conduits for exotic plant invasions in a semiarid landscape. Conservation Biology 17(2):420-432.
Gerell, R. 1997. Management of roadside vegetation: Effects on density and species diversity of butterflies in Scania, south Sweden. Entomologisk Tidskrift 118:171-176.
Ghazoul, J. 2006. Floral diversity and the facilitation of pollination. Journal of Ecology 94:295-304.
Gill, R. J. and N. E. Raine. 2014. Chronic impairment of bumblebee natural foraging behavior induced by sublethal pesticide exposure. Functional Ecology doi: 10.1111/1365-2435.12292: 1 -13.
Gill, R. J., O. Ramos-Rodriguez, and N. E. Raine. 2012. Combined pesticide exposure severely affects individual-and colony-level traits in bees. Nature doi:10.1038/nature11585.
Gjessing, E., E. Lygren, L. Berglind, T. Gulbrandsen, and R. Skanne. 1984. Effect of highway runoff on lake water quality. Science of the Total Environment 33:247-257.
Gonzalez-Terrazas, T. P., R. Medellin, M. Knörnschild, and M. Tschapka. 2012. Morphological specialization influences nectar extraction efficiency of sympatric nectar-feeding bats. Journal of Experimental Biology 215:3989-3996.
Goverde, M., K. Schweizer, B. Baur, and A. Erhardt. 2002. Small-scale habitat fragmentation effects on pollinator behaviour: experimental evidence from the bumble bee Bombus veteranus on calcareous grasslands. Biological Conservation 104:293-299.
Grant, A., C. Nelson, T. Switalski, and S. Rinehart. 2011. Restoration of native plant communities after road decommissioning in the Rocky Mountains: effect of seed-mix composition on vegetative establishment. Restoration Ecology 19:160-169.
Grant, V. 1994. Historical development of ornithophily in the western North American flora. Proceedings of the National Academy of Sciences of the United States of America 91:10407-10411.
Greenleaf, S. S., N. M. Williams, R. Winfree, and C. Kremen. 2007. Bee foraging ranges and their relationship to body size. Oecologia 153(3):589-596.
Grixti, J. C., L. T. Wong, S. A. Cameron, and C. Favret. 2009. Decline of bumble bees (Bombus) in the North American Midwest. Biological Conservation 142:75-84.
Gustafson, D. J., D. J. Gibson, and D. L. Nickrent. 2005. Using local seeds in prairie restoration—data support the paradigm. Native Plants Journal 6(1):25-28.
Haaland, C. and M. Gyllin. 2010. Butterflies and bumble bees in greenways and sown wildflower strips in southern Sweden. Journal of Insect Conservation 14(2):125-132.
Haan, N. L., M. R. Hunter, and M. D. Hunter. 2012. Investigating predictors of plant establishment during roadside restoration. Restoration Ecology 20:315-321.
Haddad, N. M. 1999. Corridor and distance effects on interpatch movements: a landscape experiment with butterflies. Ecological Applications 9:612-622.
Haddad, N. M. and K. A. Baum. 1999. An experimental test of corridor effects on butterfly densities. Ecological Applications 9:623-633.
Hansen, M. J. and A. P. Clevenger. 2005. The influence of disturbance and habitat on the presence of non-native plant species along transport corridors. Biological Conservation 125(2): 249-259.
Hanula, J. L. and S. Horn. 2011. Removing an invasive shrub (Chinese privet) increases native bee diversity and abundance in riparian forests of the southeastern United States. Insect Conservation and Diversity 4(4):275-283.
Harper, M. G., C. H. Dietrich, R. L. Larimore, and P. A. Tessene. 2000. Effects of prescribed fire on prairie arthropods: An enclosure study. Natural Areas Journal 20(4):325-335.
Harper-Lore, B., and M. Wilson, eds. 2000. Roadside Use of Native Plants. Island Press, Washington, DC, 665 pp.
Harper-Lore, B., M. Johnson, and W. F. Ostrum. 2013. Vegetation Management: An Ecoregional approach. United States Department of Transportation Federal Highway Administration.
Harrington, J. A. 1994. Roadside landscapes: Prairie species take hold in Midwest rights-of-way. Ecological Restoration 12:8-15.
Harrison, G. L. 2014. Economic Impact of Ecosystem Service Provided by Ecologically Sustainable Roadside Right of Way Vegetation Management Practices. Florida Department of Transportation Contract Number BDK75-977-74.
Hartley, M. K., W. E. Rogers, E. Siemann, and J. Grace. 2007. Responses of prairie arthropod communities to fire and fertilizer: balancing plant and arthropod conservation. American Midland Naturalist 157(1):92-105.
Hartzler, R. G. 2010. Reduction in common milkweed (Asclepias syriaca) occurrence in Iowa cropland from 1999 to 2009. Crop Protection 29: 1542-1544.
Hatfield, R. G., and G. LeBuhn. 2007. Patch and landscape factors shape community assemblage of bumble bees, Bombus spp. (Hymenoptera: Apidae), in montane meadows. Biological Conservation 139:150-158.
Hatfield, R. G., S. R. Colla, S. Jepsen, L. L. Richardson, and R. W. Thorp. 2014. In Prep. International Union for the Conservation of Nature (IUCN) Assessments for North American Bombus spp. for the North American IUCN Bumble Bee Specialist Group. The Xerces Society for Invertebrate Conservation, Portland, OR.
Hatfield, R., S. Jepsen, E. Mader, S. H. Black, and M. Shepherd. 2012. Conserving Bumble Bees, Guidelines for Creating and Managing Habitat for America's Declining Pollinators. 32 pp. Portland, OR: The Xerces Society for Invertebrate Conservation.
Hayes, G. F., and K. D. Holl. 2003. Cattle grazing impacts on annual forbs and vegetation composition of mesic grasslands in California. Conservation Biology 17(6):1694-1702.
Hines, H.. and S. D. Hendrix. 2005. Bumble bee (Hymenoptera: Apidae) diversity and abundance in tallgrass prairie patches: effects of local and landscape floral resources. Environmental Entomology 34:1477-1484.
Holm, S. N. 1966. The utilization and management of bumble bees for red clover and alfalfa seed production. Annual Review of Entomology 11:155-182.
Hopwood, J. L. 2008. The contribution of roadside grassland restorations to native bee conservation. Biological Conservation 141:2632-2640.
Hopwood, J., L. Winkler, B. Deal, and M. Chivvis. 2010. Use of roadside prairie plantings by native bees. Living Roadway Trust Fund.
Hopwood, J., M. Vaughan, E. Mader, M. Shepherd, C. Mazzacano, and S. Black. 2012. Are Neonicotinoids Killing Bees? A Review of Research into the Effects of Neonicotinoid Insecticides on Bees. 32 Pgs. The Xerces Society for Invertebrate Conservation. 628 NE Broadway, Suite 200, Portland, OR 97232
Houseal, G.. and D. Smith. 2000. Source-identified seed: The Iowa roadside experience. Ecological Restoration 18(3):173-183.
Huijser, M. P., and A. P. Clevenger. 2006. Habitat and corridor function of rights-of-way. In The ecology of transportation: managing mobility for the environment (pp. 233-254). Springer Netherlands.
Humbert, J-Y., J. Ghazoul, G. J. Sauter, and T. Walter. 2010. Impact of different meadow mowing techniques on field invertebrates. Journal of Applied Entomology 134(7):592-599.
Huntzinger, M. 2003. Effects of fire management practices on butterfly diversity in the forested western United States. Biological Conservation 113(1):1-12.
Hutson, A. M., S. P. Mickleburgh, and P. A. Racey (Ed.). 2001. Microchiropteran bats: global status survey and conservation action plan. International Union for the Conservation of Nature (IUCN) Species Survival Commission Chiroptera Specialist Group. IUCN, Gland, Switzerland and Cambridge, UK. 258 str.
Irvine, A. K, and J. E. Armstrong. 1990. Beetle pollination in tropical forests of Australia. In y K. S. Bawa and M. Hadley (Ed.), Reproductive ecology of tropical forest plants, 135-149. Paris: The Parthenon Publishing Group.
Jablonski, B., Z. Koltowski, J. Marcinkowski, H. Rybak-Chmielewska, T. Szczesna, and Z. Warakomska. 1995. Zawartosc metali ciezkich [Pb, Cd, i Cu] w nektarze, miodzie i pylku pochodzacym z roslin rosnacych przy szlakach komunikacyjnych [The content of heavy metals (Pb, Cd and Cu) in the nectar, honey and pollen collected from roadside plants]. Pszczelnicze Zeszyty Naukowe 39:129-144.
Jauker, F., T. Diekötter, F. Schwarzbach, and V. Wolters. 2009. Pollinator dispersal in an agricultural matrix: opposing responses of wild bees and hoverflies to landscape structure and distance from main habitat. Landscape Ecology 24(4):547-555.
Javorek, S. K., K. E. Mackenzie, and S. P. Vander Kloet. 2002. Comparative pollination effectiveness among bees (Hymenoptera: Apoidea) on lowbush blueberry (Ericaceae: Vaccinium angustifolium). Annals of the Entomological Society of America 95:345-351.
Jennersten, O. 1988. Pollination in Dianthus deltoides (Caryophyllaceae): effects of habitat fragmentation on visitation and seed set. Conservation Biology 2(4):359-366.
Jepsen, S., D. F. Schweitzer, B. Young, N. Sears, M. Ormes, and S. H. Black. 2015. Conservation Status and Ecology of Monarchs in the United States. 36 pp. NatureServe, Arlington, Virginia, and the Xerces Society for Invertebrate Conservation, Portland, Oregon.
Johansen, C. A. 1977. Pesticides and pollinators. Annual Review of Entomology 22:177-192.
Johansen, C. A., and D. F. Mayer. 1990. Pollinator protection: a bee and pesticide handbook. Cheshire, Connecticut: Wicwas Press.
Johnson, A. M. 2000. Best Practices Handbook on Roadside Vegetation Management. Minnesota Department of Transportation, Report # 2000-19, University of Minnesota 132 pp., p. 78. Available: https://www.lrrb.org/media/reports/200820.pdf. Accessed November 2014.
Johst, K., M. Drechsler, J. Thomas, and J. Settele. 2006. Influence of mowing on the persistence of two endangered large blue butterfly species. Journal of Applied Ecology 43(2):333-342.
Jones, A., P. Harrington, and G. Turnbull. 2014. Neonicotinoid Concentrations in Arable Soils After Seed Treatment Applications in Preceding Years. Pest Management Science doi: 10.1002/ps.3836.
Karim, M.. and A. Mallik. 2008. Roadside vegetation by native plants I. Roadside microhabitats, floristic zonation and species traits. Ecological Engineering 32:222-237.
Kearns, C. A. 2001. North American dipteran pollinators: assessing their value and conservation status. Conservation Ecology 5(1):5. Available: https://www.ecologyandsociety.org/vol5/iss1/art5/.
Kearns, C. A., and D. W. Inouye. 1997. Pollinators, flowering plants, and conservation biology. BioScience 47(5):297-307.
Kearns, C. A., and J. D. Thompson. 2001. The Natural History of Bumble bees. A Sourcebook for Investigations. Boulder: University Press of Colorado.
Kearns, C. A., D. A. Inouye, and N. M. Waser. 1998. Endangered mutualisms: the conservation of plant-pollinator interactions. Annual Review of Ecology & Systematics 29:83-113.
Kegel, B. 1989. Laboratory experiments on the side effects of selected herbicides and insecticides on the larvae of three sympatric Poecilus-species (Col., Carabidae). Journal of Applied Entomology-Zeitschrift Fur Angewandte Entomologie 108:144-155.
Kevan, P. G. 1975. Forest application of the insecticide fenitrothion and its effect on wild bee pollinators (Hymenoptera: Apoidea) of lowbush blueberries (Vaccinium spp.) in Southern New Brunswick, Canada. Biological Conservation 7(4):301-309.
Kevan, P. G. 1999. Pollinators as bioindicators of the state of the environment: species, activity and diversity. Agriculture Ecosystems & Environment 74(1-3):373-393.
Kjaer, C. and N. Elmegaard. 1996. Effect of herbicide treatment on host plant quality for a leaf-eating beetle. Pesticide Science 47:319-325.
Kjaer, C. and U. Heimbach. 2001. Relationships between sulfonylurea herbicide treatment of host plants and the performance of herbivorous insects. Pest Management Science 57:1161-1166.
Klatt, B. K., A. Holzschuh, C. Westphal Y. Clough, I. Smit, E. Pawelzik, and T. Tscharntke. 2014. Bee pollination improves crop quality, shelf life and commercial value. Proceedings of the Royal Society B: Biological Sciences 281:20132440.
Klein, A.-M., B. E. Vaissière, J. H. Cane, I. Steffan-Dewenter, S. A. Cunningham, C. Kremen, and T. Tscharntke. 2006. Importance of pollinators in changing landscapes for world crops. Proceedings of the Royal Society Series B: Biological Sciences 274:303-313.
Klein, A.-M., C. Brittain, S. D. Hendrix, R. Thorp, N. Williams, and C. Kremen. 2012. Wild pollination services to California almond rely on semi-natural habitat. Journal of Applied Ecology 49:723-732.
Koch, J. B., and J. P. Strange. 2012. The status of Bombus occidentalis and B. moderatus in Alaska with special focus on Nosema bombi incidence. Northwest Science 86(3):212-220.
Krauss, J., T. Alfert, and I. Steffan-Dewenter. 2009. Habitat area but not habitat age determines wild bee richness in limestone quarries. Journal of Applied Ecology 46:194-202.
Kremen, C., N. M. Williams, and R. W. Thorp. 2002b. Crop pollination from native bees at risk from agricultural intensification. Proceedings of the National Academy of Sciences 99(26):16812-16816.
Kremen, C., N. M. Williams, M. A. Aizen, B. Gemmill-Herren, G. LeBuhn, R. Minckley, L. Packer, S. G. Potts, T. Roulston, I. Steffan-Dewenter, D. P. Vazquez, R. Winfree, L. Adams, E. E. Crone, S.S. Greenleaf, T. H. Keitt, A. M. Klein, J. Regetz, and T. H. Ricketts. 2007. Pollination and other ecosystem services produced by mobile organisms: a conceptual framework for the effects of land-use change. Ecology Letters 10:299-314.
Kremen, C., N. M. Williams, R. L. Bugg, J. P. Fay, and R.W. Thorp. 2004. The area requirements of an ecosystem service: crop pollination by native bee communities in California. Ecology Letters 7:1109-1119.
Kremen, C., R. L. Bugg, N. Nicola, S. A. Smith, R. W. Thorp, and N. M. Williams. 2002a. Native bees, native plants, and crop pollination in California. Fremontia 30(3-4):41-49.
Krewenka, K. M., A. Holzschuh, T. Tscharntke, and C. F. Dormann. 2011. Landscape elements as potential barriers and corridors for bees, wasps and parasitoids. Biological Conservation 144(6), 1816-1825.
Kruess, A., and T. Tscharntke. 2002a. Contrasting responses of plant and insect diversity to variation in grazing intensity. Biological Conservation 106(3):293-302.
Kruess, A., and T. Tscharntke. 2002b. Grazing intensity and the diversity of grasshoppers, butterflies, and trap-nesting bees and wasps. Conservation Biology 16:1570-80.
Krupke, C. H., G. J. Hunt, B. D. Eitzer, G. Andino, and K. Given. 2012. Multiple routes of pesticide exposure for honey bees living near agricultural fields. PLOS ONE 7(1), e29268. DOI: 10.1371/journal.pone.0029268.
Kutlesa, N. J. and S. Caveney. 2001. Insecticidal activity of glufosinate through glutamine depletion in a caterpillar. Pest Management Science 57:25-32.
LaBar, C. C. and C. B. Schultz. 2012. Investigating the role of herbicides in controlling invasive grasses in prairie habitats: effects on non-target butterflies. Natural Areas Journal 32(2):177-189.
Larson, B. M. H., P. G. Kevan, and D. W. Inouye. 2001. Flies and flowers: taxonomic diversity of anthophiles and pollinators. The Canadian Entomologist 133:439-465.
Leharne, S., D. Charesworth, and B. Chowdhry. 1992. A survey of metal levels in street dusts in an inner London neighbourhood. Environment International 18:263-270.
Lentini, P. E., T. G. Martin, P. Gibbons, J. Fischer, and S. A. Cunningham. 2012. Supporting wild pollinators in a temperate agricultural landscape: maintaining mosaics of natural features and production. Biological Conservation 149(1):84-92.
Linsley, E. G. 1958. The ecology of solitary bees. Hilgardia 27:543-599.
Lippit, L., M. W. Fidelibs, and D. A. Bainbridge. 1994. Native seed collection, processing, and storage for revegetation projects in the western United States. Restoration Ecology 2:120-131.
Losey, J. E. and M. Vaughan. 2006. The economic value of ecological services provided by insects. Bioscience 56:311-323.
Louda, S. M. and C. W. O'Brien. 2002. Unexpected ecological effects of distributing the exotic weevil, Larinus planus (F.), for the biological control of Canada thistle. Conservation Biology 16(3):717-727.
Louda, S. M., C. Kendall, J. Connor, and D. Simberloff. 1997. Ecological effects of an insect introduced for the biological control of weeds. Science 277:1088-1090.
Lövei, G. L., A. Macleod, and J. M. Hickman. 1998. Dispersal and effects of barriers on the movement of the New Zealand hover fly Melanostoma fasciatum (Dipt., Syrphidae) on cultivated land. Journal of Applied Entomology 122(1-5), 115-120.
Lucey, A. and S. Barton. 2011. Influencing public perception of sustainable roadside vegetation management strategies. Journal of Environmental Horticulture 29(3):119-124.
Mader, E., J. Hopwood, L. Morandin, M. Vaughan, and S. H. Black. 2014. Farming with native beneficial insects: Ecological Pest Control Solutions. Storey Publishing, 272 pp.
Mader, E., M. Spivak, and E. Evans. 2010. Managing Alternative Pollinators: A Handbook for Beekeepers, Growers, and Conservationists. Sustainable Agriculture Research and Education, Handbook 11. College Park, MD: University of Maryland, Sustainable Agriculture Research and Extension and Ithaca, NY: Cornell University, Natural Resource, Agriculture, and Engineering Service.
Marks, R. 2006. Native Pollinators. U.S. Department of Agriculture Natural Resources Conservation Service. Fish and Wildlife Habitat Management Leaflet. No. 34.
Marrs, R. H., A. J. Frost, R. A. Plant, and P. Lunnis. 1993. Determination of buffer zones to protect seedlings of non-target plants from the effects of glyphosate spray drift. Agriculture, Ecosystems & Environment 45(3):283-293.
McCain, K. N. S., S. G. Baer, J. M. Blair, and G. W. T. Wilson. 2010. Dominant grasses suppress local diversity in restored tallgrass prairie. Restoration Ecology 18:40-49.
McFrederick, Q. S., J. C. Kathilankal, and J. D. Fuentes. 2008. Air pollution modifies floral scent trails. Atmospheric Environment 42(10):2336-2348.
McKenna, D. D., K. M. McKenna, S. B. Malcom, and M. R. Berenbaum. 2001. Mortality of Lepidoptera along roadways in central Illinois. Journal of the Lepidopterists Society 55(2):63-68.
Medler, J. T. and D. W. Carney. 1963. Bumble bees of Wisconsin (Hymenoptera: Apidae). Research Bulletin, University of Wisconsin, Agricultural Experiment Station 240:47 pp.
Memmott, J. and N. M. Waser. 2002. Integration of alien plants into a native flower-pollinator visitation web. Proceedings of the Royal Society of London Series B: Biological Sciences 269:2395-2399.
Memmott, J., N. M. Waser, and M. V. Price. 2004. Tolerance of pollination networks to species extinctions. Proceedings of the Royal Society of London Series B: Biological Sciences 271:2605-2611.
Menz, M. H., R. D. Phillips, R. Winfree, C. Kremen, M. A. Aizen, S. D. Johnson, and K. W. Dixon. 2011. Reconnecting plants and pollinators: challenges in the restoration of pollination mutualisms. Trends in Plant Science 16(1):4-12.
Michener, C. D. 2007. The Bees of the World, 2nd Ed. 992 pp. Baltimore: John Hopkins University Press.
Middleton, E. L., J. D. Bever, and P. A. Schultz. 2010. The effect of restoration methods on the quality of the restoration and resistance to invasion by exotics. Restoration Ecology 18:181-187.
Miller, S. A., A. Bartow, M. Gisler, K. Ward, A. S. Young, and T. N. Kaye. 2011. Can an ecoregion serve as a seed transfer zone? Evidence from a common garden study with five native species. Restoration Ecology 19:268-276.
Minnesota Department of Natural Resources. 2014. Roadsides for Wildlife. Available: http://www.dot.state.mn.us/roadsides/vegetation/.
Mitchell, T. B. 1962. Bees of the Eastern United States. Vol. II. North Carolina Agricultural Experiment Station Technical Bulletin 152:1-557.
Monroe, M., D. Frey, and S. Stevens. 2014. Western Monarch Thanksgiving Count Data from 1997-2017. Available: https://www.westernmonarchcount.org/data/.
Montgomery, D. P., D. L. Martin, and C. C. Evans. 2010. Oklahoma Roadside Vegetation Management Guidelines, 4th Edition. Available: http://pods.dasnr.okstate.edu/docushare/dsweb/Get/Document-3158/Roadside+Vegetation.pdf.
Morandin, L. A. and C. Kremen. 2013a. Bee preference for native versus exotic plants in restored agricultural hedgerows. Restoration Ecology 21(1):26-32.
Morandin, L. A. and C. Kremen. 2013b. Hedgerow restoration promotes pollinator populations and exports native bees to adjacent fields. Ecological Applications 23(4):829-839.
Morandin, L. A. and M. L. Winston. 2006. Pollinators provide economic incentive to preserve natural land in agroecosystems. Agriculture, Ecosystems & Environment 116(3):289-292.
Morandin, L. A., M. L. Winston, M. T. Franklin, and V. A. Abbott. 2005. Lethal and sub-lethal effects of spinosad on bumble bees (Bombus impatiens Cresson). Pest Management Science 61:619-626.
Moroń, D., H. Szentgyorgyi, I. Grzes', M. Wantuch, E. Rozej, R. Laskowski and M. Woyciechowski. 2010. The effect of heavy metal pollution on development of wild bees. Atlas of Biodiversity Risks - From Europe to the Globe, From Stories to Maps (eds J. Settele, L.D. Penev, T.A. Georgiev, R. Grabaum, V. Grobelnik, V. Hammen et al.), pp. 224-225. Pensoft, Sofia & Moscow.
Moroń, D., I. M. Grześ, P. Skórka, H. Szentgyörgyi, R. Laskowski, S. G. Potts, and M. Woyciechowski. 2012. Abundance and diversity of wild bees along gradients of heavy metal pollution. Journal of Applied Ecology 49(1):118-125.
Morris, M. G. 1967. Differences between the invertebrate faunas of grazed and ungrazed chalk grassland. I. Responses of some phyto-phagous insects to cessation of grazing. Journal of Applied Ecology 4:459-474.
Morris, M. G. 2000. The effects of structure and its dynamics on the ecology and conservation of arthropods in British grasslands. Biological Conservation 95:121-226.
Morse, R. A. and N. W. Calderone. 2000. The value of honey bees as pollinators of US crops in 2000. Bee Culture 128:1-15.
Mulder, C., T. Aldenberg, D. de Zwart, H. J. van Wijnen, and A. M. Breure. 2005. Evaluating the impact of pollution on plant-Lepidoptera relations. Environmetrics 16:357-373.
Munguira, M. L. and J. A. Thomas. 1992. Use of road verges by butterfly and burnet populations, and the effect of roads on adult dispersal and mortality. Journal of Applied Ecology 29:316-329.
Nabhan, G. P., R. C. Brusca, and L. Holter, ed. 2004. Conserving migratory pollinators and nectar corridors in western North America. Tucson: University of Arizona Press.
National Research Council. 2007. Status of Pollinators in North America. Washington, D.C.: National Academies Press.
National Resources Conservation Service. 2013. Web Soil Survey. U.S. Department of Agriculture. Available: https://websoilsurvey.nrcs.usda.gov/app/.
NatureServe. 2014. Conservation Status. Available: https://www.natureserve.org/conservation-tools/conservation-status-assessment.
Ne'eman, G., A. Dafni, and S. G. Potts. 2000. The effect of fire on flower visitation rate and fruit set in four core-species in east Mediterranean scrubland. Plant Ecology 146:97-104.
Nebraska Weed Control Association (NWCA) and Nebraska Department of Agriculture. 2014. Thistles of Nebraska. Available: http://www.neweed.org/Documents/Thistles%20of%20Nebraska.pdf. Accessed November 2014.
Nieminen, M., N. Pekka, and E. Tulisalo. 2001. The effect of metals on the mortality of Parnassius apollo larvae (Lepidoptera: Papilionidae). Journal of Insect Conservation 5(1):1-7.
Noordijk, J., K. Delille, A. P. Schaffers, and K. V. Sýkora. 2009. Optimizing grassland management for flower-visiting insects in roadside verges. Biological Conservation 142:2097-2103.
O'Dell, R. E., S. L. Young, and V. P. Claassen. 2007. Native roadside perennial grasses persist a decade after planting in the Sacramento Valley. California Agriculture 61(2):79-84.
O'Toole, C. and A. Raw. 1999. Bees of the World, 192 pp. London: Blandford.
Oakley, C. and J. Knox. 2013. Plant species richness increases resistance to invasion by non-resident plant species during grassland restoration. Applied Vegetation Science 16:21-28.
Oberts, G. L. 1986. Pollutants associated with sand and silt applied to roads in Minnesota. Water Resources Bulletin 22:479-483.
Öckinger, E. and H. G. Smith. 2008. Do corridors promote dispersal in grassland butterflies and other insects? Landscape Ecology 23(1):27-40.
Ollerton, J., R. Winfree, and S. Tarrant. 2011. How many flowering plants are pollinated by animals? Oikos 120:321-326.
Osborne, J. L. and I. H. Williams. 2001. Site constancy of bumble bees in an experimentally patchy habitat. Agriculture, Ecosystems & Environment 83:129-141.
Osborne, J. L., A. P. Martin, N. L. Carreck, J. L. Swain, M. E. Knight, D. Goulson, R. J. Hale, and R. A. Sanderson. 2008. Bumble bee flight distances in relation to the forage landscape. Journal of Animal Ecology 77(2):406-415.
Ottewell, K. M., S. C. Donnellan, A. J. Lowe, and D. C. Paton. 2009. Predicting reproductive success of insect-versus bird-pollinated scattered trees in agricultural landscapes. Biological Conservation 142(4):888-898.
Ouin, A., S. Aviron, J. Dover, and F. Burel. 2004. Complementation/supplementation of resources for butterflies in agricultural landscapes. Agriculture, Ecosystems & Environment 103:473-479.
Packard, S. and C. F. Mutel. 1997. The Tallgrass Restoration Handbook: for prairies, savannas, and woodlands. Washington, DC: Island Press.
Panzer, R. 2002. Compatibility of prescribed burning with the conservation of insects in small, isolated prairie reserves. Conservation Biology 16:1296-1307.
Parr, T. W. and J. M. Way. 1988. Management of roadside vegetation: the long-term effects of cutting. Journal of Applied Ecology 25:1073-1087.
Pemberton, R. W. 2000. Predictable risk to native plants in weed biological control. Oecologia 125(4):489-494.
Perugini, M., M. Manera, L. Grotta, M. Cesarina Abete, R. Tarasco, and M. Amorena. 2011. Heavy metal (Hg, Cr, Cd, and Pb) contamination in urban areas and wildlife reserves: honeybees as bioindicators. Biological Trace Element Research 140(2):170-176.
Pickles, W. 1942. Animal mortality on three miles of Yorkshire roads. The Journal of Animal Ecology: 37-43.
Pleasants, J. M. and K. S. Oberhauser. 2012. Milkweed loss in agricultural fields because of herbicide use: effect on the monarch butterfly population. Insect Conservation and Diversity 6:135-144.
Potts, S. G., B. Vulliamy, A. Dafni, G. Ne'eman, and P. G. Willmer. 2003b. Linking bees and flowers: how do floral communities structure pollinator communities? Ecology 84:2628-2642.
Potts, S. G., B. Vulliamy, A. Dafni, G. Ne'eman, C. O'Toole, S. Roberts, and P. Willmer. 2003a. Response of plant-pollinator communities to fire: changes in diversity, abundance and floral reward structure. Oikos 101(1):103-112.
Potts, S. G., B. Vulliamy, S. Roberts, C. O'Toole, A. Dafni, G. Ne'eman, and P. G. Willmer. 2005. Role of nesting resources in organizing diverse bee communities in a Mediterranean landscape. Ecological Entomology 30(1):78-85.
Potts, S. G., J. C. Biesmeijer, C. Kremen, P. Neumann, O. Schweiger, and W. E. Kunin. 2010. Global pollinator declines: trends, impacts and drivers. Trends in Ecology and Evolution 25(6):345-353.
Procter, M., P. Yeo, and A. Lack. 1996. The Natural History of Pollination. Portland: Timber Press.
Purtauf, T., I. Roschewitz, J. Dauber, C. Thies, T. Tscharntke, and V. Wolters. 2005. Landscape context of organic and conventional farms: Influences on carabid beetle diversity. Agriculture, Ecosystems & Environment 108:165-174.
Pywell, R. F., E. A. Warman, C. Carvell, T. H. Sparks, L. V. Dicks, D. Bennett, A. Wright, C. N. R. Critchley, and A. Sherwood. 2005. Providing foraging resources for bumble bees in intensively farmed landscapes. Biological Conservation 121:479-494.
Quales, W. 2003. Native plants and integrated roadside vegetation management. IPM Practitioner 25(3-4): 1-9.
Quarles III, H. D., R. B. Hanawalt, and W. E. Odum. 1974. Lead in small mammals, plants, and soil at varying distances from a highway. Journal of Applied Ecology: 937-949.
Rao, R. S. P. and M. K. S. Girish. 2007. Road kills: Assessing insect casualties using flagship taxon. Current Science 92(6):830-843.
Rasmont, P., A. Pauly, M. Terzo, S. Patiny, D. Michez, S. Iserbyt, Y. Barbier, and E. Haubruge. 2006. The survey of wild bees (Hymenoptera, Apoidea) in Belgium and France. In Status of the World's Pollinators. Rome: United Nations Food and Agriculture Organization.
Ravenscroft, N. O. M. 1994. The ecology of the chequered skipper butterfly Carterocephalus palaemon in Scotland. I. Microhabitat. Journal of Applied Ecology: 613-622.
Reese, N. E. 1977. Impact of Rhinocyllus conicus on thistles in southwestern Montana. Environmental Entomology 6:839-842
Rendón-Salinas, E. and G. Tavera-Alonso. 2014. Monitoreo de la superficie forestal ocupada por las colonias de hibernación de la mariposa Monarca en diciembre de 2013. Alianza WWF-Telcel / CONANP. 5 pp. Available: https://wwflac.awsassets.panda.org/downloads/mmonarca.pdf.
Rentch, J. S., R. H. Fortney, S. L. Stephenson, H. S. Adams, W. N. Grafton, and J. T. Anderson. 2005. Vegetation-site relationships of roadside plant communities in West Virginia, USA. Journal of Applied Ecology 42(1):129-138.
Richardson, S. 2005. Lesser Long-nosed Bat - Five-Year Review. U.S. Fish and Wildlife Service, Phoenix, Arizona. Available: https://www.fws.gov/southwest/es/arizona/Documents/SpeciesDocs/LLNB/LLNB_5yr_Final.pdf.
Ricketts, T. H., J. Regetz, S. A. Cunningham, C. Kremen, A. Bogdanski, B. Gemmill-Herren, S. S. Greenleaf, A.-M. Klein, M. M. Mayfield, L. A. Morandin, A. Ochieng, S. G. Potts, and B. F. Viana. 2008. Landscape effects on crop pollination services: are there general patterns? Ecology Letters 11:499-515.
Ries, L. and D. M. Debinski. 2001. Butterfly responses to habitat edges in the highly fragmented prairies of Central Iowa. Journal of Animal Ecology 70(5):840-852.
Ries, L., D. M. Debinski, and M. L. Wieland. 2001. Conservation value of roadside prairie restoration to butterfly communities. Conservation Biology 15:401-411.
Russell, C. and C. B. Schultz. 2010. Investigating the use of herbicides to control invasive grasses: effects on at-risk butterflies. Journal of Insect Conservation 14(1):53-63.
Russell, K. N., H. Ikerd, and S. Droege. 2005. The potential conservation value of unmowed powerline strips for native bees. Biological Conservation 124:133-148.
Saarinen, K., A. Valtonen, J. Jantunen, and S. Saarnio. 2005. Butterflies and diurnal moths along road verges: Does road type affect diversity and abundance? Biological Conservation 123:403-412.
Samways, M. J., P. M. Caldwell, and R. Osborn. 1996. Ground-living invertebrate assemblages in native, planted and invasive vegetation in South Africa. Agriculture, Ecosystems & Environment 59:19-32.
Sandrock, C., L. G. Tanadini, J. S. Pettis, J. C. Biesmeijer, S. G. Potts, and P. Neumann. 2013. Sublethal neonicotinoid insecticide exposure reduces solitary bee reproductive success. Agricultural and Forest Entomology. doi: 10.1111/afe.12041.
Santamour, F. S. Jr. 1990. Trees for Urban Planning: Diversity, Uniformity, and Common Sense. Proceedings of the. 7th Metropolitan Tree Improvement Alliance Conference. 7:57-65.
Saunders, D. A., R. J. Hobbs, and C. R. Margules. 1991. Biological consequences of ecosystem fragmentation - a review. Conservation Biology 5:18-32.
Schaffers, A. P., I. P. Raemakers, and K. V. Sykora. 2012. Successful overwintering of arthropods in roadside verges. Journal of Insect Conservation 16(4):511-522.
Schneider, C. W., J. Tautz, B. Grünewald, and S. Fuchs. 2012. RFID tracking of sublethal effects of two neonicotinoid insecticides on the foraging behavior of Apis mellifera. PLOS ONE 7(1): e30023. doi:10.1371/journal.pone.0030023.
Schramm, P. 1990. Prairie restoration: a twenty-five year perspective on establishment and management. In D. D. Smith and C. A. Jacobs (eds.), Recapturing a vanishing heritage. Proceedings of the Twelfth North American Prairie Conference, University of Northern Iowa, Cedar Falls, IA.
Scott, J. 1986. The Butterflies of North America: A Natural History and Field Guide. Stanford University Press, 584 pp.
Seibert, H. C. and J. H. Conover. 1991. Mortality of vertebrates and invertebrates on an Athens County, Ohio, highway. Ohio Journal of Science 91(4):163-166.
Severns, P. M. 2008. Exotic grass invasion impacts fitness of an endangered prairie butterfly, Icaricia icarioides fenderi. Journal of Insect Conservation 12(6):651-661.
Shigo, A. 1991. Modern Arboriculture: A systems approach to the care of trees and their associates. Durham, NH: Shigo and Trees, Associates.
Simberloff, D. and P. Stiling. 1996. How risky is biological control? Ecology 77(7):1965-1974.
Skórka, P., M. Lenda, D. Moroń, K. Kalarus, and P. Tryjanowski. 2013. Factors affecting road mortality and the suitability of road verges for butterflies. Biological Conservation 159:148-157.
Slayback, D. A., L. P. Brower, M. I. Ramirez, and L. S. Fink. 2007. Establishing the presence and absence of overwintering colonies of the monarch butterfly in Mexico by the use of small aircraft. American Entomologist 53:28-40.
Smallidge, P. J. and D. J. Leopold. 1997. Vegetation management for the maintenance and conservation of butterfly habitats in temperate human-dominated habitats. Landscape and Urban Planning 38:259-280.
Smallidge, P. J., D. J. Leopold, and C. M. Allen. 1996. Community characteristics and vegetation management of Karner blue butterfly (Lycaeides melissa samuelis) habitats on rights-of-way in east-central New York, USA. Journal of Applied Ecology, 1405-1419.
Smith, L. 2006. Enhanced Biological Control of Yellow Starthistle and Tumbleweed (Russian Thistle). California Department of Transportation Report CA06-0282. Available: http://www.dot.ca.gov/research/researchreports/2002-2006/2006/task_0282-design_and_construction.pdf.
Soderstrom, B. and M. Hedblom. 2007. Comparing movement of four butterfly species in experimental grassland strips. Journal of Insect Conservation 11(4):333-342.
Speight, M. C. D. 1978. Flower-visiting flies. In A Dipterist's Handbook edited by A. Stubbs, P. Chandler, and P. W. Cribb, 229-236. The Amateur Entomologists' Society.
Spellerberg, I. 1998. Ecological Effects of Roads and Traffic: A Literature Review. Global Ecology and Biogeography Letters 7(5):317-333.
Spira, T. P. 2001. Plant-pollinator interactions: a threatened mutualism with implications for the ecology and management of rare plants. Natural Areas Journal 21(1):78-88.
Stark, J.D., X. D. Chena, and C. S. Johnson. 2012. Effects of herbicides on Behr's metalmark butter?y, a surrogate species for the endangered butter?y, Lange's metalmark. Environmental Pollution 164:24-27.
Steffan-Dewenter, I. and C. Westphal. 2008. The interplay of pollinator diversity, pollination services and landscape change. Journal of Applied Ecology 45(3):737-741.
Steffan-Dewenter, I. and S. Schiele. 2008. Do resources or natural enemies drive bee population dynamics in fragmented habitats? Ecology 89:1375-1387.
Steinfeld, D., S. Riley, K. Wilkinson, T. Landis, and L. Riley. 2007. Roadside Revegetation: An Integrated Approach to Establishing Native Plants. Federal Highway Administration. Western Federal Lands Highway Division. Report No. FHWA-WFL/TD-07-005.
Stienauer, G. 2003. A Guide to Prairie and Westland Restoration in Eastern Nebraska. Prairie Plains Resource Institute and Nebraska Game and Parks Commission.
Stoner, K. J. L. and A. Joern. 2004. Landscape vs. local habitat scale influences to insect communities from tallgrass prairie remnants. Ecological Applications 14:1306-1320.
Stubbs, A. and P. Chandler, eds. 1978. A Dipterist's Handbook. Amateur Entomologist 15:1-255.
Sugden, E. A. 1985. Pollinators of Astragalus monoensis Barneby (Fabaceae): new host records; potential impact of sheep grazing. Great Basin Naturalist 45:299-312.
Sutcliffe, O. L. and C. D. Thomas. 1996. Open corridors appear to facilitate dispersal by ringlet butterflies (Aphantopus hyperantus) between woodland clearings. Conservation Biology 10(5) :1359-1365.
Svensson, B., J. Lagerlöf, and B. G. Svensson. 2000. Habitat preferences of nest-seeking bumble bees (Hymenoptera: Apidae) in an agricultural landscape. Agriculture, Ecosystems & Environment 77(3):247-255.
Swaileh, K. M., R. M. Hussein, and S. Abu-Elhaj. 2004. Assessment of heavy metal contamination in roadside surface soil and vegetation from the West Bank. Archives of Environmental Contamination and Toxicology 47(1):23-30.
Swengel, A. B. 2001. A literature review of insect responses to fire, compared to other conservation managements of open habitat. Biodiversity and Conservation 10:1141-1169.
Szentgyörgyi, H., A. Blinov, N. Eremeeva, S. Luzyanin, I. M. Grześ, and M. Woyciechowski. 2011. Bumble bees (Bombidae) along pollution gradient - heavy metal accumulation, species diversity, and Nosema bombi infection level. Polish Journal of Ecology 59,:599-610.
Tallamy, D. W. and K. J. Shropshire. 2009. Ranking lepidopteran use of native versus introduced plants. Conservation Biology 23(4):941-947.
Tan, K., C. Weiwen, D. Shihao, L. Xiwen, W. Yuchong, and C. N. James. 2014. Imidacloprid Alters Foraging and Decreases Bee Avoidance of Predators. PLOS ONE 9(7): e102725. DOI: 10.1371/journal.pone.0102725
Tepedino, V. J. 1981. The pollination efficiency of the squash bee (Peponapis pruinosa) and the honey bee (Apis mellifera) on summer squash (Cucurbita pepo). Journal of the Kansas Entomological Society: 359-377.
Tewksbury, J. J, D. J. Levey, N. M. Haddad, S. Sargent, J. L. Orrock, A. Weldon, B. J. Danielson, J. Brinkerhoff, E. I. Damschen, and P. Townsend. 2002. Corridors affect plants, animals, and their interactions in fragmented landscapes. Proceedings of the National Academy of Sciences 99(20): 12923-12926.
Thomas, C. D. and T. M. Jones. 1993. Partial recovery of a skipper butterfly (Hesperia comma) from population refuges: lessons for conservation in a fragmented landscape. Journal of Animal Ecology: 472-481.
Thomas, J. A. 1984. Conservation of butterflies in temperate countries: past efforts and lessons for the future. In Symposia of the Royal Entomological Society of London.
Thomas, J. A., R. G. Snazell, and L. K. Ward. 2002. Are roads harmful or potentially beneficial to butterflies and other insects? In B. Sherwood, D. Cutler, and J. A. Burton (eds.), Wildlife and roads: the ecological impact (pp. 203-222). London: Imperial College Press.
Thompson, H. M. 2003. Behavioural effects of pesticides use in bees - their potential for use in risk assessment. Ecotoxicology 12:317-330.
Thorp, R. W. 2003. Bumble Bees (Hymenoptera: Apidae): Commercial use and environmental concerns. pp. 21-40. In K. Strickler and J. H. Cane (eds.). For nonnative crops, whence pollinators of the future? Thomas Say Publications in Entomology: Proceedings. Entomological Society of America, Lanham, MD.
Tilman, D., P. B. Reich, and J. M. H. Knops. 2006. Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature 441:629-632.
Tinsley, J. M., M. T. Simmons, and S. Windhager. 2006. The establishment success of native versus non-native herbaceous seed mixes on a revegetated roadside in Central Texas. Ecological Engineering 26:231-240.
Townsend, P. A. and D. J. Levey. 2005. An experimental test of whether habitat corridors affect pollen transfer. Ecology 86(2):466-475.
Transportation Research Board. 2004. Environmental Stewardship Practices, Procedures, and Policies for Highway Construction and Maintenance. National Cooperative Highway Research Program Project 25-25 (04). Available: https://apps.trb.org/cmsfeed/TRBNetProjectDisplay.asp?ProjectID=761.
Transportation Research Board. 2005. Integrated Roadside Vegetation Management. National Cooperative Highway Research Program. Synthesis 341. Available: http://www.trb.org/Publications/Blurbs/155791.aspx.
Trombulak, S. C. and C. A. Frissell. 2000. Review of ecological effects of roads on terrestrial and aquatic communities. Conservation Biology 14(1):18-30.
Tscharntke, T., A. Gathmann, and I. Steffan-Dewenter. 1998. Bioindication using trap-nesting bees and wasps and their natural enemies: community structure and interactions. Journal of Applied Ecology 35:708-719.
Tyser, R. W. and C. A. Worley. 1992. Alien flora in grasslands adjacent to road and trail corridors in Glacier National Park, Montana (USA). Conservation Biology 6(2):253-262.
Tyser, R. W., J. M. Asebrook, R. W. Potter, and L. L. Kurth. 1998. Roadside revegetation in Glacier National Park, USA: effects of herbicide and seeding treatments. Restoration Ecology 6(2):197-206.
U.S. and Wildlife Service. 2014. Environmental Conservation Online System. Listed Animals. October 20, 2014. Available: https://ecos.fws.gov/ecp0/reports/ad-hoc-species-report?kingdom=V&kingdom=I&status=E&status=T&status=EmE&status=EmT&status=EXPE&status=EXPN&status=SAE&status=SAT&fcrithab=on&fstatus=on&fspecrule=on&finvpop=on&fgroup=on&header=Listed+Animals.
U.S. Department of Agriculture, Forest Service. 2006. Cooperating Across Boundaries. Partnerships to Conserve Open Space in Rural America. Cooperative Forestry FS-861.
U.S. Fish and Wildlife Service. 2006. Lesser long-nosed bat (Leptonycteris curasoae yerbabuenae) 5-year review: summary and evaluation. U.S. Fish and Wildlife Service, Phoenix, Arizona.
Valiente-Banuet, A., F. Molina-Freaner, A. Torres, M. C. Arizmendi, and A. Casas. 2004. Geographic differentiation in the pollination system of the columnar cactus Pachycereus pecten-aboriginum. American Journal of Botany 91:850-855.
Valtonen, A. and K. Saarinen. 2005. A highway intersection as an alternative habitat for a meadow butterfly: effect of mowing, habitat geometry and roads on the ringlet (Aphantopus hyperantus). Annales Zoologici Fennici 42(5):545-556.
Valtonen, A., K. Saarinen, and J. Jantunen. 2006. Effect of different mowing regimes on butterflies and diurnal moths on road verges. Animal Biodiversity and Conservation 29:133-148.
Valtonen, A., K. Saarinen, and J. Jantunen. 2007. Intersection reservations as habitats for meadow butterflies and diurnal moths: Guidelines for planning and management. Landscape and Urban Planning 79(3):201-209.
Van Geert, A., F. Van Rossum, and L. Triest. 2010. Do linear landscape elements in farmland act as biological corridors for pollen dispersal? Journal of Ecology 98(1):178-187.
Van Nuland, M. E., E. N. Haag, J. A. Bryant, Q. D. Read, R. N. Klein, M. J. Douglas, C. E. Gorman, T. D. Greenwell, M. B. Busby, J. Collins, J. T. LeRoy, G. Schuchmann, J. A. Schweitzer, and J. K. Bailey. 2013. Fire promotes pollinator visitation: Implications for ameliorating declines of pollination services. PlOS ONE, 8(11), e79853. doi:10.1371/journal.pone.0079853.
Vance, N. C., A. Neill, and F. Morton. 2006. Native grass seedling and forb planting establishment in a degraded oak savanna in the Coast Range foothills of western Oregon. Native Plants Journal 7(2):35-46.
Vinchesi, A. C. 2014. Assessing transportation impacts to alkali bees (Hymenoptera: Halictidae) and alfalfa seed production in the Walla Walla Valley. PhD dissertation, Washington State University. Available: http://media.proquest.com/media/pq/classic/doc/3428092951/fmt/ai/rep/NPDF?_s=gqqgK0oFXH%2F35qE%2B1SAg0KM%2FWOs%3D. Accessed October 2014.
Vogel, J. A., R. R. Koford, and D. M. Debinski. 2010. Direct and indirect responses of tallgrass prairie butterflies to prescribed burning. Journal of Insect Conservation 14(6):663-677.
Von der Lippe, M. and I. Kowarik. 2007. Long?Distance Dispersal of Plants by Vehicles as a Driver of Plant Invasions. Conservation Biology 21(4):986-996.
Vulliamy, B., S. G. Potts, and P. G. Willmer. 2006. The effects of cattle grazing on plant-pollinator communities in a fragmented Mediterranean landscape. Oikos 114:529-543.
Warren, M. S. 1993. A review of butterfly conservation in central southern Britain: II. Site management and habitat selection of key species. Biological Conservation 64(1):37-49.
Washington State Department of Transportation. 2014. Roadside Manual. Available: http://www.wsdot.wa.gov/publications/manuals/fulltext/M25-30/Roadside.pdf. Accessed November 2014.
Way, J. M. 1977. Roadside verges and conservation in Britain: a review. Biological Conservation 12:65-74.
Westerkamp, C. and G. Gottsberger. 2000. Diversity pays in crop pollination. Crop Science 40:1209-1222.
Westrich, P. 1996. Habitat requirements of central European bees and the problems of partial habitats. In A. Matheson, S. L. Buchmann, C. O'Toole, P. Westrich, and I. H. Williams (eds.), Conservation of Bees. London: Academic Press.
Whitehorn, P. R., S. O'Connor, F. L. Wackers, and D. Goulson. 2012. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336(6079):351-352.
Williams, D. W., L. L. Jackson, and D. D. Smith. 2007. Effects of frequent mowing on survival and persistence of forbs seeded into a species-poor grassland. Restoration Ecology 15:24-33.
Williams, N. M. and C. Kremen. 2007. Resource distribution among habitats determine solitary bee offspring production in a mosaic landscape. Ecological Applications 17:910-921.
Williams, N. M., D. Cariveau, R. Winfree, and C. Kremen. 2011. Bees in disturbed habitats use, but do not prefer, alien plants. Basic and Applied Ecology 12(4):332-341.
Williamson, P. and P. R. Evans. 1972. Lead: levels in roadside invertebrates and small mammals. Bulletin of Environmental Contamination and Toxicology 8(5):280-288.
Wilson, D. E. and D. M. Reeder (Eds). 2005. Mammal Species of the World: A Taxonomic and Geographic Reference, third edition. Baltimore, MD: Johns Hopkins University Press.
Winfree, R., N. M. Williams, H. Gaines, J. S. Ascher, and C. Kremen. 2008. Wild bee pollinators provide the majority of crop visitation across land-use gradients in New Jersey and Pennsylvania, USA. Journal of Applied Ecology 45(3):794-802.
Winfree, R., R. Aguilar, D. P. Vázquez, G. LeBuhn, and M. A. Aizen. 2009. A meta-analysis of bees' responses to anthropogenic disturbance. Ecology 90:2068-2076.
Wu, Y-T., C.-H. Wang, X.-D. Zhang, B. Zhao, L.-F. Jiang, J.-K. Chen, and B. Li. 2009. Effects of saltmarsh invasion by Spartina alterniflora. Biological Invasions 11:635-649.
Wynhoff, I. 1998. Lessons from the reintroduction of Maculinea teleius and M. nausithous in the Netherlands. Journal of Insect Conservation 2 (1):47-57.
Wynhoff, I., R. Van Gestel, C. Van Swaay, and F. Van Langevelde. 2011. Not only the butterflies: managing ants on road verges to benefit Phengaris (Maculinea) butterflies. Journal of Insect Conservation 15(1-2):189-206.
Xerces Society Red List of Pollinator Species. 2007.
Zielin, S., C. E. de Rivera, S. Jacobson, and W. P. Smith. 2010. Exploring mitigation options to reduce vehicle-caused mortality of a threatened butterfly. Transportation Research Board Annual Meeting. Available: http://itre.ncsu.edu/ADC30/11_TRB_Winter_Conference/Presentations/Paper_11-2834.pdf. Accessed October 2014.
Zuefle, M. E., W. P. Brown, and D. W. Tallamy. 2008. Effects of non-native plants on native insect community of Delaware. Biological Invasions 10:1159-1169.
7.2 Personal Communications
Dr. Jaret Daniels, University of Florida; Dr. Karen Oberhauser, University of Minnesota; Dr. Art Shapiro, University of California Davis; Dr. John Shuey, Chair of the Lepidopterists' Society's Conservation Committee.
Back to top

